Buparvaquone preparation method
A technology of bupavaquinone and compounds, applied in the field of drug synthesis, can solve the problems of high price of acetaldehyde, low product purity, poor stability, etc., and achieve cheap and easy-to-obtain raw materials, high purity and yield, and stable reaction intermediates Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
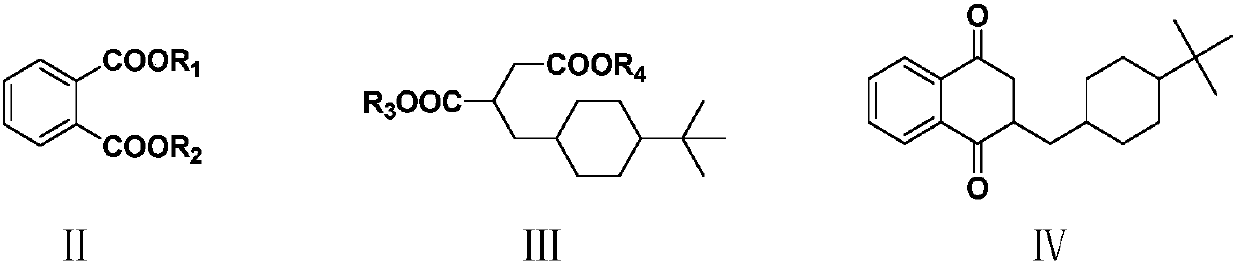
[0066] Example 1: Preparation of 2-[(4-tert-butylcyclohexyl)methyl]-2,3-dihydro-1,4-naphthalenedione (IV)
[0067] Into a 500 ml four-neck flask connected with stirring, thermometer, reflux condenser and dropping funnel, add 200 g of tetrahydrofuran, 13.5 g (0.25 mol) of sodium methoxide, dropwise add 19.4 g (0.1 mol) ) a mixture of dimethyl phthalate (II1), 29.8 grams (0.1 mole) of 2-(4-tert-butylcyclohexylmethyl) dimethyl succinate (III1) and 50 grams of tetrahydrofuran, dropwise in 2 hours, Thereafter the reaction was stirred at 45 to 50°C for 4 hours. Cool to 20 to 25°C, add 200 g of water, heat, and stir the hydrolysis reaction at 65 to 70°C for 3 hours. Cool to 30 to 35°C, acidify the system with 30wt% hydrochloric acid to pH 1.0-2.0, decarboxylate at 30 to 40°C for 1 hour, cool to 20 to 25°C, add 200 g of dichloromethane, extract, separate layers, water layer Extract 3 times with dichloromethane, each 30 grams, combine organic phase, 50 grams of 5wt% sodium bicarbonat...
Embodiment 2
[0068] Example 2: Preparation of 2-[(4-tert-butylcyclohexyl)methyl]-2,3-dihydro-1,4-naphthalenedione (IV)
[0069] To a 500 ml four-necked flask connected with stirring, a thermometer, a reflux condenser and a dropping funnel, add 200 g of toluene, 24.6 g (0.22 moles) of potassium tert-butoxide, between 70 and 75 ° C, dropwise add 22.2 g ( A mixture of 0.1 mol) diethyl phthalate (Ⅱ2), 32.6 g (0.1 mol) 2-(4-tert-butylcyclohexylmethyl) diethyl succinate (Ⅲ2) and 50 g of toluene, drop in 2 hours After that, the reaction was stirred at 75 to 80°C for 4 hours thereafter. Cool to 20 to 25°C, add 200 g of water, heat, and stir the hydrolysis reaction at 65 to 70°C for 3 hours. Cool to 30 to 35°C, acidify with 30wt% hydrochloric acid until the pH of the system is 1.0-2.0, decarboxylate at 30 to 40°C for 1 hour, cool to 20 to 25°C, separate layers, extract the water layer with toluene 3 times, each time 30 grams, combined organic phase, 50 grams of 5wt% sodium bicarbonate aqueous sol...
Embodiment 3
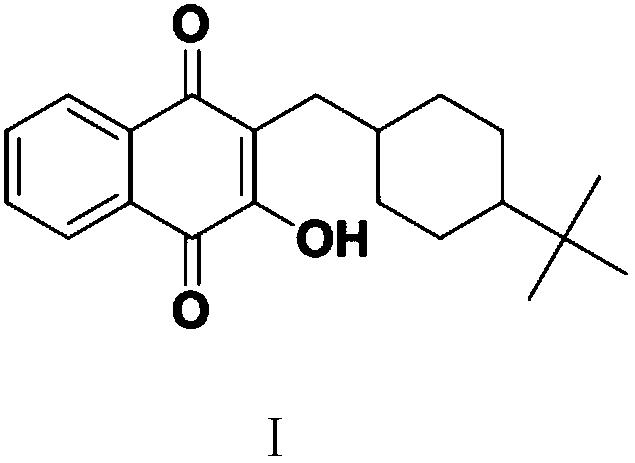
[0070] Embodiment 3: the preparation of bupavaquinone (I)
[0071] In a 500 ml four-neck flask connected with stirring, a thermometer, a reflux condenser and a constant pressure dropping funnel, add 60 grams of 1,2-dichloroethane, 15.6 grams (0.05 moles) of 2-[ (4-tert-butylcyclohexyl)methyl]-2,3-dihydro-1,4-naphthalenedione (IV), add 16.8 grams (0.105 moles) of bromine and 30 grams of 1,2 - The mixture of dichloroethane, about 1 hour dripping, thereafter, 30-35 ° C stirring reaction for 3 hours, cooled to 20-25 ° C, added 50.0 grams (0.25 moles) 20wt% sodium hydroxide aqueous solution, 50-55 ° C Stir the reaction for 2 hours to eliminate hydrogen bromide, then stir the hydrolysis reaction at 75-80°C for 2 hours, cool to 20-25°C, acidify the system with 30wt% hydrochloric acid to a pH value of 6.0-7.0, separate layers, and use 1,2-bis Extract 3 times with ethyl chloride, 30 grams each time, combine the organic phases, distill and recover 1,2-dichloroethane, add 0.3 grams of a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com