Lithium cobalt iron phosphate lithium ion battery positive electrode material and preparation method thereof
A lithium ion battery, lithium iron cobalt phosphate technology, applied in battery electrodes, positive electrodes, secondary batteries, etc., can solve the problems of low redox potential, poor ionic conductivity, low rate performance, etc., to improve rate performance, improve Electrical conductivity, the effect of improving power density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
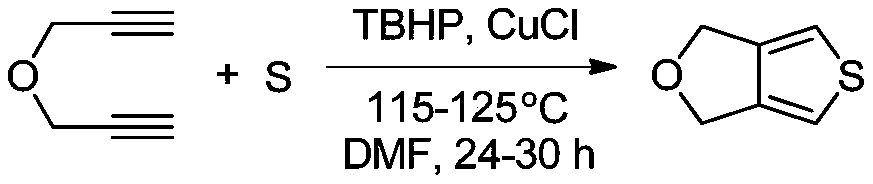
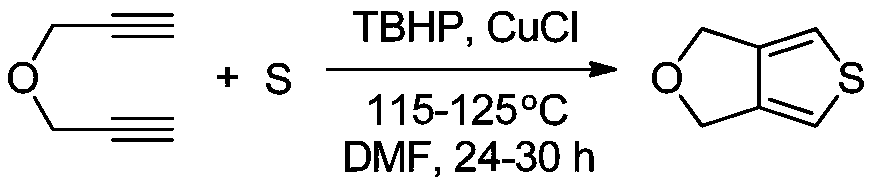
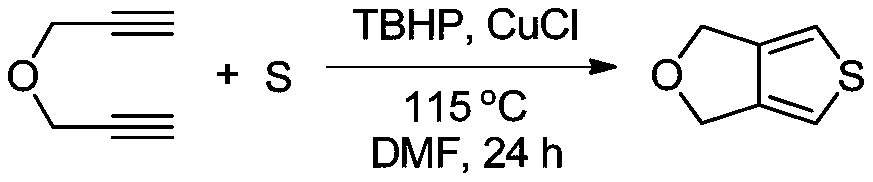
[0022] The molecular formula of tetrahydrofurothiazole is C 6 h 6 OS, the structural formula is The preparation method comprises the following steps:
[0023] (1) Pass N into the three-necked bottle 2 Exclude the air, then add 200-800mL of anhydrous N,N-dimethylformamide (DMF), then add propargyl ether and sulfur element in turn and stir evenly, then add tert-butanol peroxide (TBHP) and chlorinated Cuprous, the molar ratio of the four substances is 1:3-5:1.2-1.5:0.1-0.3, the three-necked bottle is placed in a constant temperature oil bath, heated to 115-125°C, and stirred at a constant speed for 24- 30h, the reaction was observed by TLC thin-layer chromatography. After the reaction of propargyl ether was complete, the solution was transferred into a separatory funnel, and distilled water and ethyl acetate were added successively to extract 3-5 times, and ethyl acetate was taken. The organic phase is concentrated under reduced pressure by a rotary evaporator, and the conce...
Embodiment 1
[0030] (1) Preparation of tetrahydrofurothiazole Inject N into the three-necked flask 2 Exclude the air, then add 200mL of anhydrous N,N-dimethylformamide (DMF), then add propargyl ether and sulfur element in turn and stir evenly, then add tert-butanol peroxide (TBHP) and cuprous chloride , the molar ratio of the four substances is 1:3:1.2:0.1, the three-necked bottle is placed in a constant temperature oil bath, heated to 115 ° C, stirred at a constant speed for 24 hours, and the reaction is observed by TLC thin layer chromatography. As a result, when the reaction of propargyl ether was complete, the solution was transferred into a separatory funnel, and distilled water and ethyl acetate were added successively for extraction 3 times. The organic phase of ethyl acetate was concentrated under reduced pressure by a rotary evaporator, and the concentrated mixture was passed through silica gel. The chromatographic column is separated by thin-layer chromatography, and the eluent...
Embodiment 2
[0035] (1) Preparation of tetrahydrofurothiazole Inject N into the three-necked flask 2 Exclude the air, then add 200mL of anhydrous N,N-dimethylformamide (DMF), then add propargyl ether and sulfur element in turn and stir evenly, then add tert-butanol peroxide (TBHP) and cuprous chloride , the molar ratio of the four substances is 1:3.5:1.3:0.15, the three-necked bottle is placed in a constant temperature oil bath, heated to 115 ° C, stirred at a constant speed for 26 hours, and the reaction is observed by TLC thin layer chromatography. As a result, when the reaction of propargyl ether was complete, the solution was transferred into a separatory funnel, and distilled water and ethyl acetate were added successively for extraction 3 times. The organic phase of ethyl acetate was concentrated under reduced pressure by a rotary evaporator, and the concentrated mixture was passed through silica gel. The chromatographic column is separated by thin-layer chromatography, and the elu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com