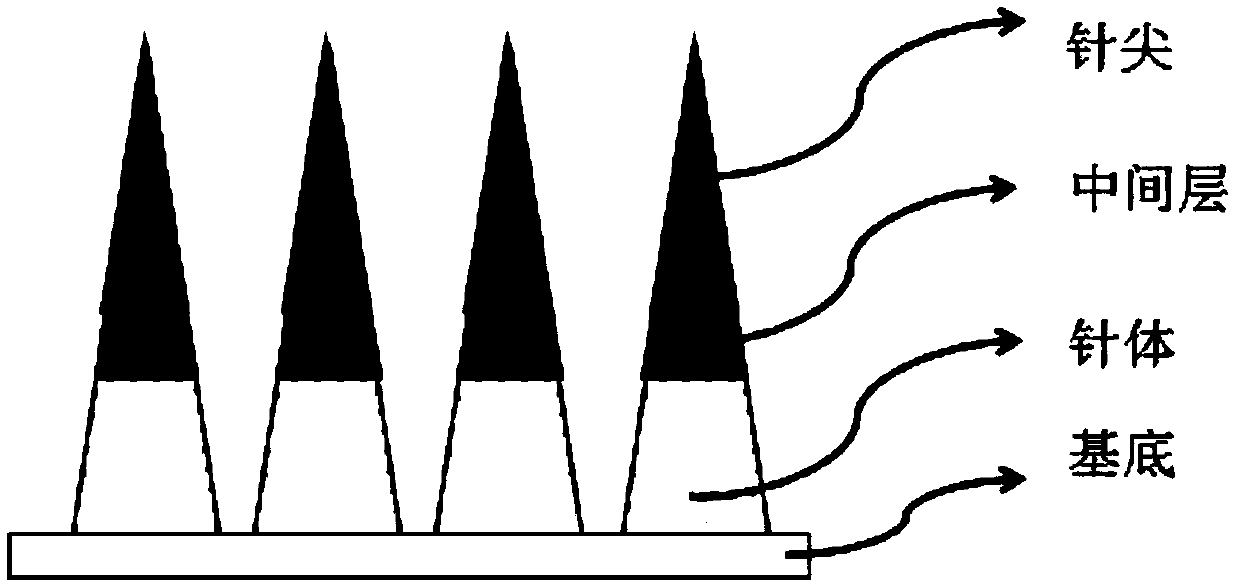
Fast-implantable sustained-release microneedle patch and preparation method of fast-implantable sustained-release microneedle
A micro-needle and sustained-release technology, applied in the field of medicine, can solve the problems of complex process, cumbersome operation and high cost, and achieve the effects of wide range of drugs, reduction of process cost, and increase of drug load.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1 Preparation of rapidly implantable sustained-release microneedles
[0049] 1. Weigh 0.3g of PLGA (80 / 20) with a molecular weight of 20kDa, add 0.7ml N-methylpyrrolidone, and prepare a PLGA solution with a mass fraction of 30%; weigh 100mg of etonogestrel, add 1ml of N-methylpyrrolidone Pyrrolidone, be mixed with the etonogestrel solution of 100mg / ml; Above-mentioned etonogestrel solution 0.5ml is mixed with above-mentioned PLGA solution 0.5ml, is mixed with the 15% PLGA solution containing etonogestrel (50mg / ml), as needle tip injection Mold fluid.
[0050] 2. Weigh 0.25g of polyvinylpyrrolidone with a molecular weight of 10kDa, add 4.75ml of water to prepare an aqueous solution of 5% polyvinylpyrrolidone by mass fraction as the intermediate layer injection molding solution; weigh 3.5g of polyvinyl alcohol with a molecular weight of 50kDa. Add 6.5ml of water, heat at 80°C for 2 hours, and prepare a polyvinyl alcohol aqueous solution with a mass fraction of...
Embodiment 2
[0059] Embodiment 2 Preparation of rapidly implantable sustained-release microneedles
[0060] 1. Weigh 0.4g of polylactic acid (PLA) with a molecular weight of 10kDa, add 0.6ml of N-methylpyrrolidone to prepare a PLA solution with a mass fraction of 40%; weigh 10mg of red fluorescent dye, add 1ml of N-methylpyrrolidone , be prepared into a fat-soluble analog drug red fluorescent dye solution with a mass fraction of 10 mg / ml; mix 0.5 ml of the red fluorescent dye solution with 0.5 ml of the PLA solution, and prepare a 20% PLA solution containing the red fluorescent dye 5 mg / ml, As a needle tip injection molding fluid.
[0061] 2. Weigh 0.05g of pullulan, add 9.95ml of water, and prepare a pullulan aqueous solution with a mass fraction of 0.5%, as the intermediate layer injection molding solution; weigh 2.5g of sodium carboxymethyl cellulose with a molecular weight of 200kDa, add 7.5ml of water was prepared as an aqueous solution of 25% sodium carboxymethyl cellulose in mass f...
Embodiment 3
[0067] Example 3 Preparation of Rapidly Implantable Sustained Release Microneedles
[0068] 1. Weigh 0.3g of PLGA (75 / 25) with a molecular weight of 10kDa, add 0.7ml N-methylpyrrolidone, and prepare a PLGA solution with a mass fraction of 30%; weigh 15mg of interferon a-2b and dissolve it in sterile water for injection , add 35 mg of zinc hydroxide after micronization and sterilization, vortex and mix for 10 minutes to form 1 g of zinc salt interferon a-2b solution; add 1 g of zinc salt interferon a-2b solution to the above 30% PLGA The solution is stirred to form a drug-loaded sol system, which is used as a needle tip injection molding solution.
[0069] 2. Weigh 0.25g of trehalose, add 4.75ml of water to make an aqueous solution of trehalose with a mass fraction of 5%, and use it as the intermediate layer injection molding solution; weigh 3g of polyvinyl alcohol with a molecular weight of 70kDa, add 7ml of water, and place Heat at 85° C. for 2 hours, and prepare an aqueous ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com