Synthesis method of zinc complex, and applications of zinc complex as fluorescent probe and ferroelectric material
A fluorescent probe, zinc complex technology, applied in luminescent materials, zinc organic compounds, material analysis by optical means, etc., to achieve the effect of high yield, easy separation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The synthesis of embodiment 1 complex:
[0034] 14.8 mg of zinc nitrate hexahydrate (Zn(NO 3 ) 2 ·6H 2 O), 18.7mg of 1,1'-(4-carboxyphenyl)-2,2'-biimidazole (H 2 L), 7.81 mg of 4,4'-bipyridine (4,4'-Bipyridine) were dissolved in 10 mL of water, and then added at a concentration of 0.15 mol L -1 hydrochloric acid solution to adjust the pH to 1.2, stirred for half an hour, transferred to the polytetrafluoroethylene liner of a 25mL hydrothermal reactor, and reacted for 72 hours at a temperature of 100°C. The resulting product was washed three times with ethanol (2mL / time), A colorless transparent bulk crystal was obtained. The calculated yield based on Zn metal was 45.3%.
Embodiment 2
[0035] The structural characterization of embodiment 2 complex:
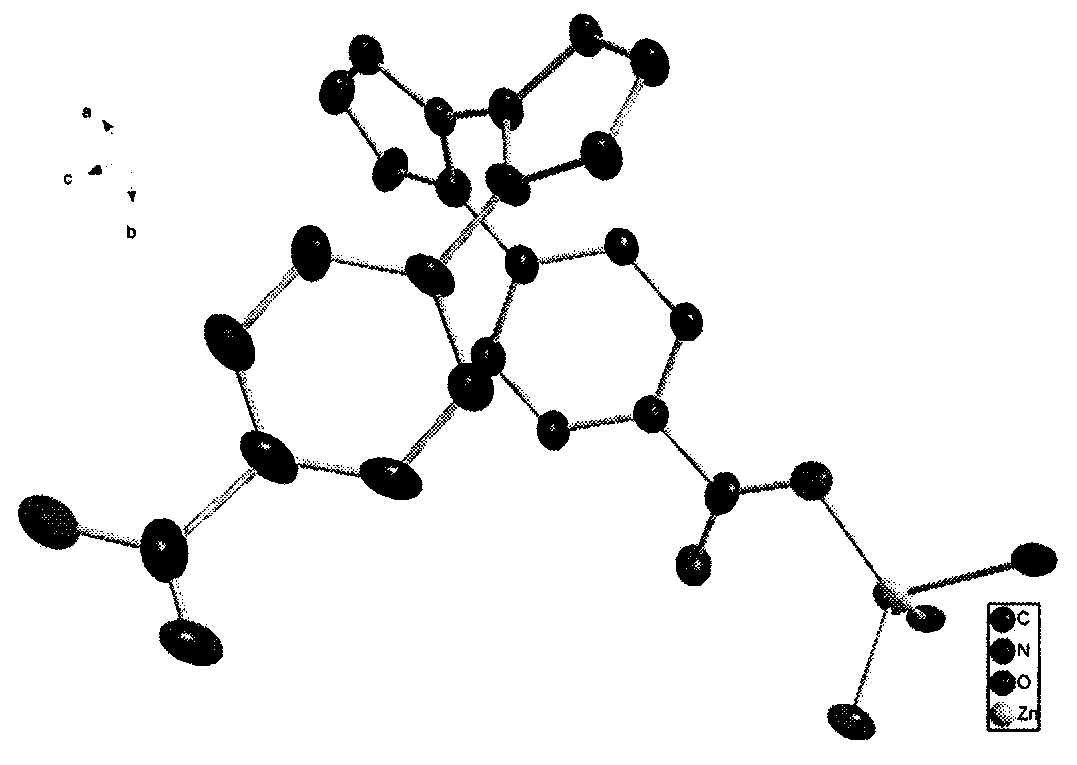
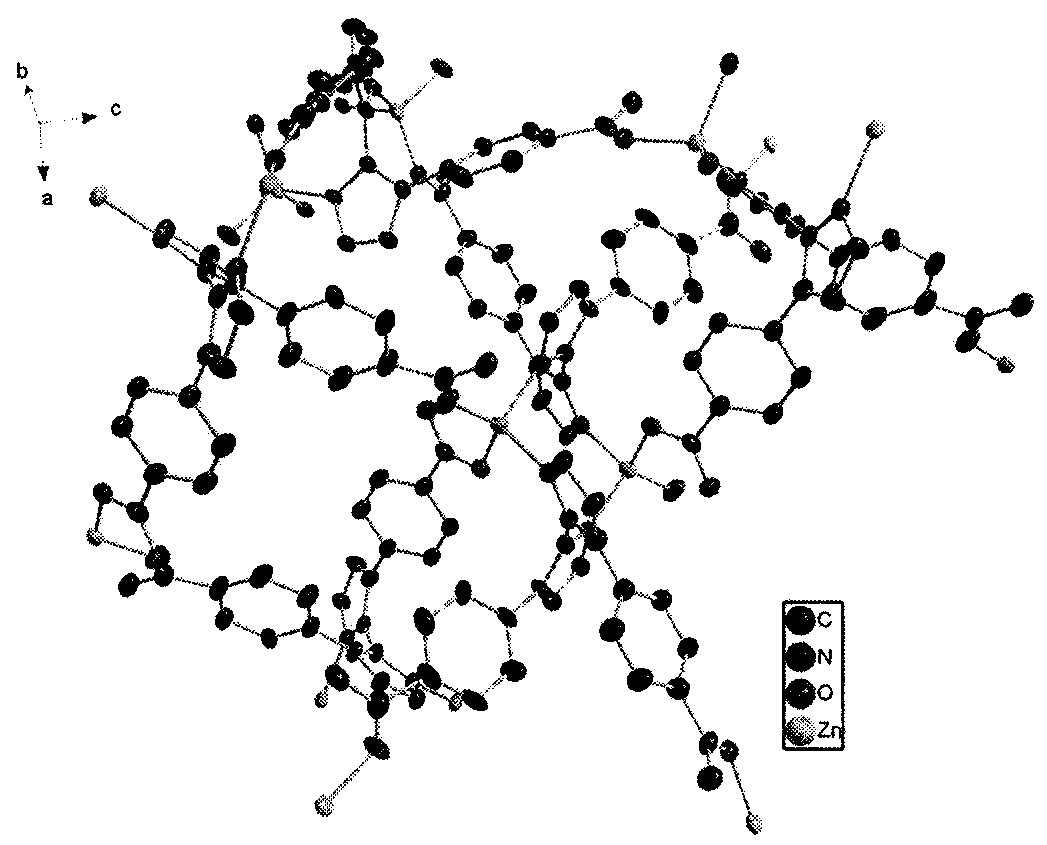
[0036] The crystal structure adopts Bruker Smart CCD X-ray single crystal diffractometer, and at 296(2) K, selects the crystal with the size of 0.31×0.14×0.13mm, and uses the MoKα ray (λ=0.07107nm) after graphite monochromatization as The incident radiation source is used to collect diffraction points by ω / 2θ scanning, the unit cell parameters are refined by the least square method, and the collected data is corrected for absorption with the SADABS program. The structure of the complex was solved by the direct method, the coordinates of non-hydrogen atoms and the anisotropy temperature factor were refined by the full-matrix least-squares method, and all calculations were completed by the SHELXTL program. The detailed crystallographic parameters are listed in Table 1. [Zn(L)·H 2 O] n The crystal structure diagram and three-dimensional structure diagram are shown in Fig. 1(a) and Fig. 1(b).
[0037] Crystallog...
Embodiment 3
[0040] The fluorescent property of embodiment 3 complexes:
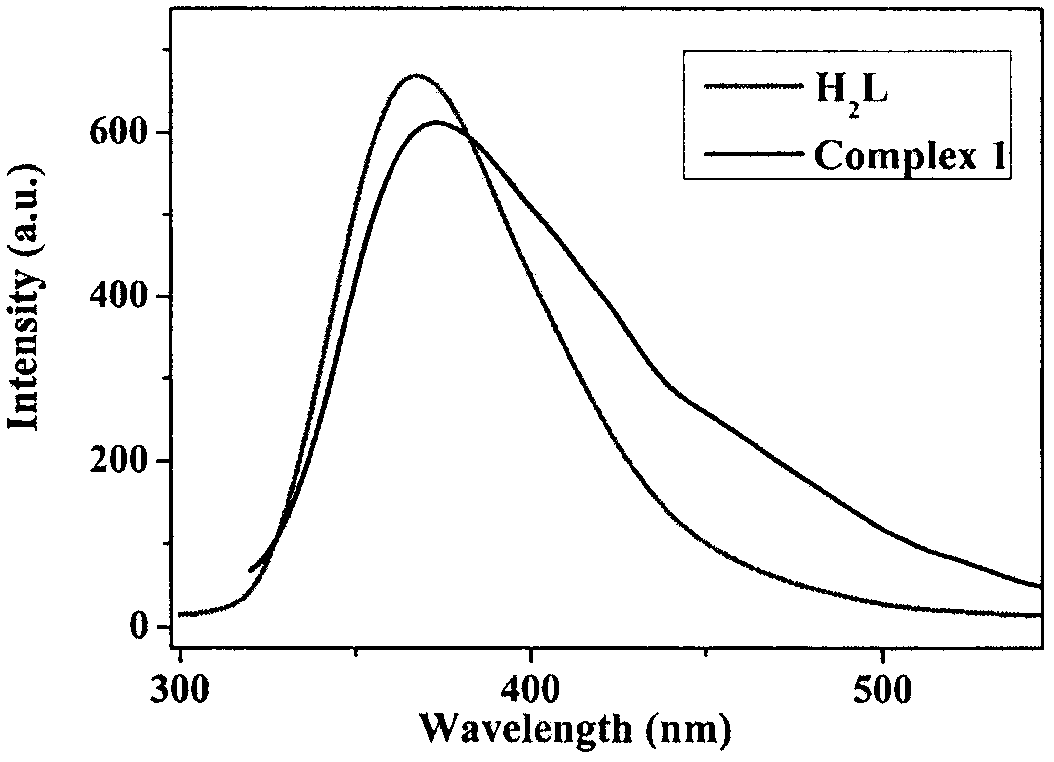
[0041] LS-55 fluorescence spectrometer was used to test the fluorescence properties of the complexes at room temperature. figure 2 It is the solid-state fluorescence image of the zinc complex prepared in Example 1, indicating that the material has strong fluorescence properties. Such as image 3 As shown, the complex exhibits strong luminescent properties in aqueous solution.
[0042] It can be seen from Fig. 4(a) that in the selected metal ion aqueous solution, the fluorescence intensity of the zinc complex prepared in Example 1 shows a dependence on the metal ion, and on Fe 3+ exhibits a complete fluorescence quenching effect and is expected to become a Fe 3+ fluorescent probes.
[0043] It can be seen from Figure 5(a) that in the selected anion aqueous solution, the fluorescence intensity of the zinc complex prepared in Example 1 shows a dependence on the type of anion, only Cr 2 o 7 2- exhibits a complete ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com