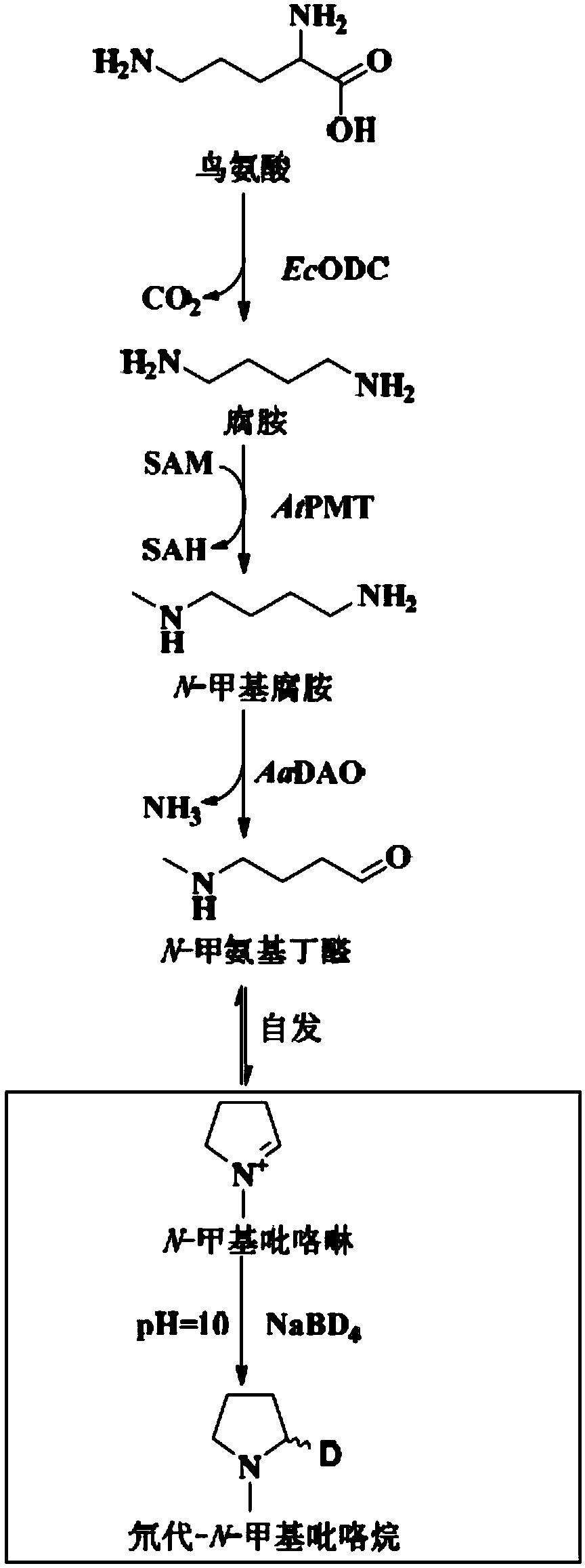
Method for preparing N-methyl pyrroline
A technology of methylpyrroline and microorganisms, which is applied in the fields of biocatalysis and metabolic engineering, and can solve problems such as large environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1 Preparation of enzymes related to N-methylpyrroline biosynthetic pathway
[0060] 1. Preparation of Ornithine Decarboxylase EcODC
[0061] 1.1 Using the EcODC coding gene (SEQ ID NO: 1) synthesized by Suzhou Jinweizhi Biotechnology Co., Ltd. as a template, PCR amplification was performed with KOD DNA polymerase (94°C for 2 min; 98°C for 10s, 55°C for 30s, 68°C for 1min; 30 cycles; 68°C for 7min), and use the Axygen Gel Extraction Kit (AxyPrep DNAGelExtraction Kit) to recover the target DNA fragment.
[0062] 1.2 Use Thermofisher’s NdeI and NotI double enzyme digestion gel to recover fragments and pET-24a(+) (Novagen), digest at 37°C for 2 hours, use Axygen Company’s AxyPrep PCR Cleanup Kit to clean and recover the digested products, and recover from Axygen gel Kit (AxyPrep DNA Gel Extraction Kit kit) gel recovery target fragments. T4 DNA ligase from NEB Company was used for ligation at 20°C for 2h, the ligation system was added to Top10 competent cells, i...
Embodiment 2
[0072] Example 2 AaDAO2, AaDAO3 activity test and in vitro mixed enzyme reaction synthesis of N-methylpyrroline
[0073] 2.1 Amine oxidase AaDAO2 and AaDAO3 activity experiment (100 microliters):
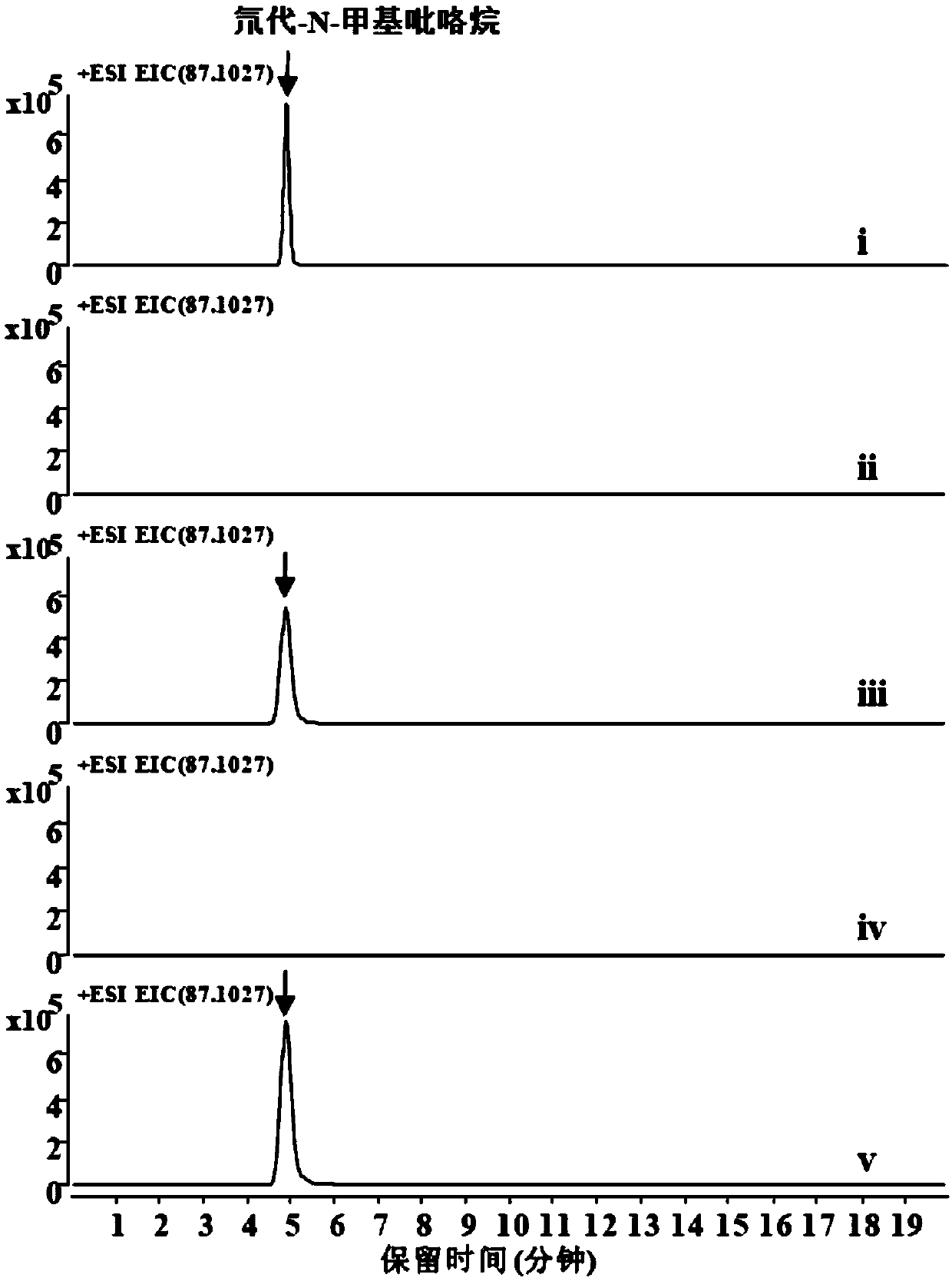
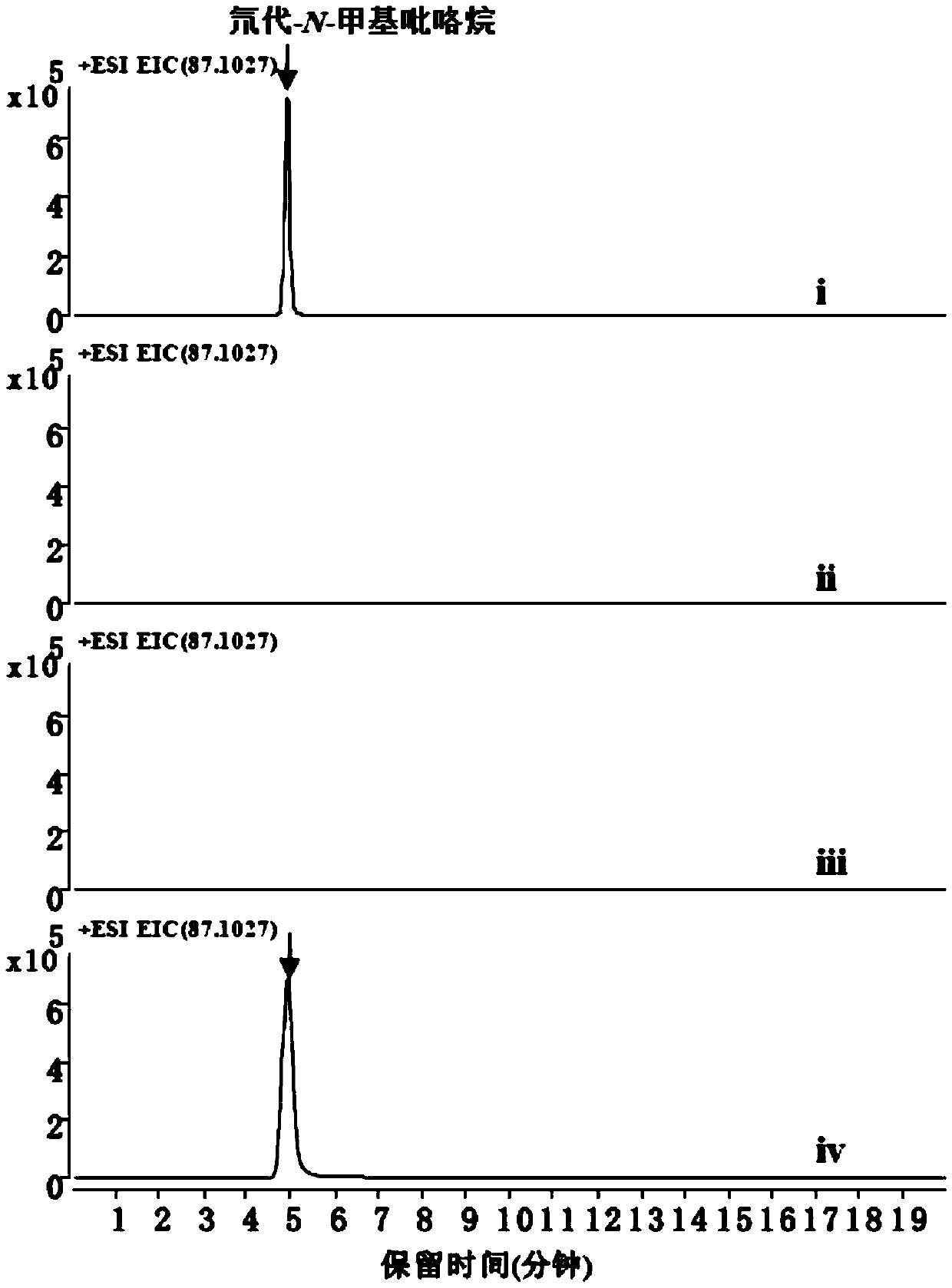
[0074] 2 mM substrate N-methylated putrescine, 20 μM CuSO 4 , 50mM potassium phosphate (pH 8.0), enzyme protein 10μM, make up with water to form a 100μl system, and react at 30°C for 30min. Add 10 µl NaBD 4 (Mother solution 1M dissolved in 0.1M sodium borate buffer, pH 10.0) reduction. Liquid phase-mass spectrometry detected that N-methylpyrroline can be reduced to generate N-methylpyrrolidine, and the molecular weight of the target compound is 87.1027. Such as Figure 2A As shown, the experimental results show that both AaDAO2 and AaDAO3 can catalyze N-methylated putrescine to generate N-methylpyrroline.
[0075] 2.2 EcODC, AtPMT and AaDAO3 mixed enzyme reaction (100 microliters):
[0076] 5 mM substrate L-ornithine, 5 mM PLP (pyridoxal phosphate), 5 mM SAM (S-adenosylmethion...
Embodiment 3
[0077] Example 3 Construction and fermentation of E. coli engineering bacteria producing N-methylpyrroline
[0078] 3.1 Using the coding gene of EcODC (SEQ ID NO: 1) as a template, PCR amplification was carried out with KOD DNA polymerase (94°C 2min; 98°C 10s, 55°C 30s, 68°C for 1min; 30 cycles; 68°C for 7min). Agarose gel electrophoresis to recover the target fragment.
[0079] 3.2 The recovered fragment and pACYCDuet-1 plasmid were digested with BamHI and HindIII, digested at 37°C for 2 hours, the digested product was cleaned and recovered, the digested pACYCDuet-1 plasmid was recovered by gel, ligated with T4 DNA ligase at 20°C for 2 hours. Add E.coli TOP10 competent state to the connection system, ice bath for 10min, heat shock at 42°C for 1min30s, place on ice for 5min, add 1mL LB, incubate at 37°C, 200rpm shaker for 45min. Centrifuge at 12,000 rpm for 1 min, discard 800 microliters of the supernatant, and coat the remaining 200 microliters on a solid LB plate containin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com