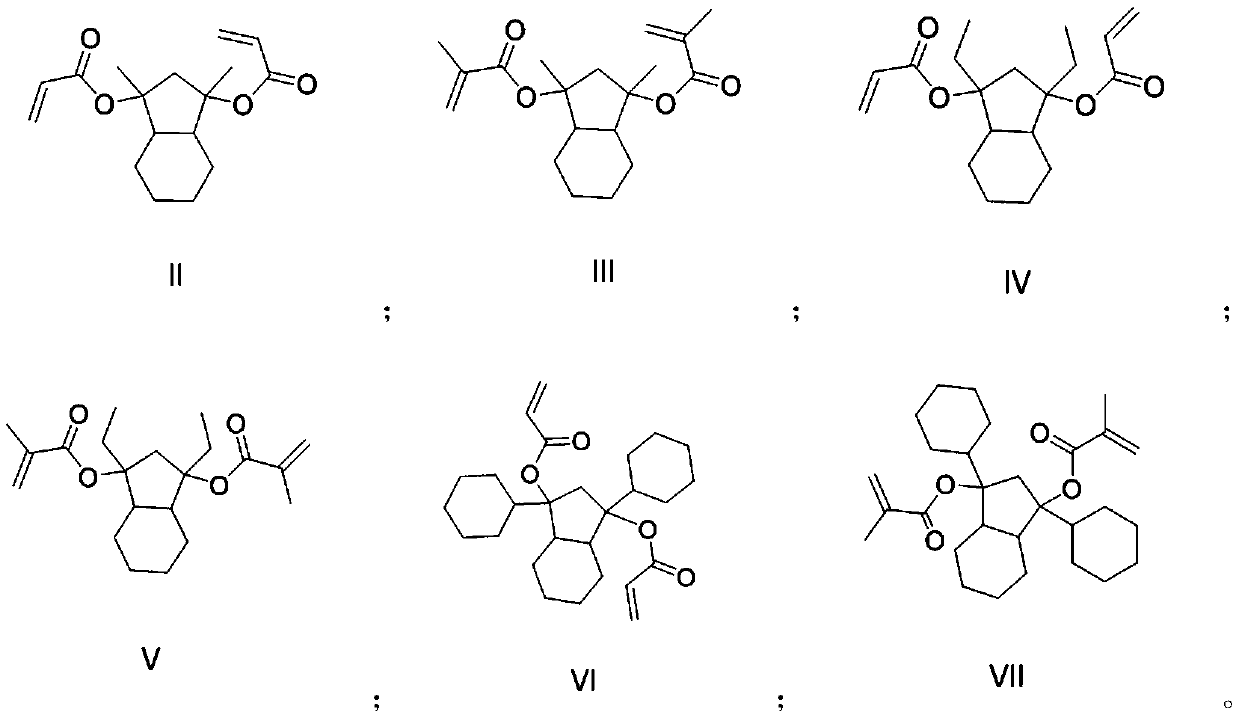
Photoresist resin monomer synthesized from hexahydro-1H-indene-1,3 (2H)-dione, and synthesis method thereof
A technology of resin monomer and synthesis method, applied in the field of photoresist resin monomer and its synthesis, can solve the problem of low resolution of photolithography pattern, and achieve the problem of improving resolution, increasing solubility, and increasing the difference in dissolution rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
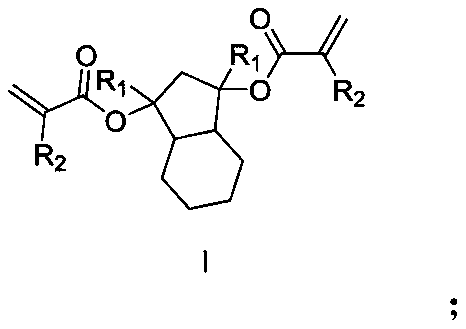
[0032] This embodiment provides a photoresist resin monomer synthesized by hexahydro-1H-indene-1,3(2H)-dione, and the reaction scheme of the synthesis method of the photoresist resin monomer is as follows:
[0033]
[0034] It specifically includes the following steps:
[0035] S1, preparation of methyl Grignard reagent: first add 3.2g of magnesium chips (132mmol) to 15ml of anhydrous ether, and add an iodine tablet to obtain a reaction solution; then, dissolve 12.5g of methyl bromide (132mmol) in 25mL Prepare a methyl bromide ether solution in diethyl ether; then, under the protection of nitrogen, first add 6mL methyl bromide ether solution to the above reaction solution. After 5 minutes, the reaction solution boils slightly, and the color of iodine disappears. Under stirring, continue to Add the remaining methyl bromide ethyl ether solution dropwise, and add 20 mL of diethyl ether, raise the temperature to keep boiling slightly, and reflux for half an hour to obtain the m...
Embodiment 2
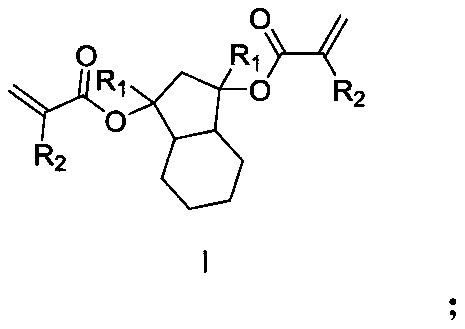
[0039] This embodiment provides a photoresist resin monomer synthesized by hexahydro-1H-indene-1,3(2H)-dione, and the reaction scheme of the synthesis method of the photoresist resin monomer is as follows:
[0040]
[0041] It specifically includes the following steps:
[0042] S1. According to the steps S1-S2 of the above-mentioned Example 1, 10.2 g of the intermediate (Formula 2-2, 55 mmol) was obtained, and the yield was 84.2%.
[0043]S2. Dissolve 10.2g of the above intermediate in 120mL of anhydrous tetrahydrofuran, and add 22.4g of triethylamine (221mmol); then, cool to 0 degrees Celsius with ice water, and slowly drop into it under the protection of nitrogen Add a solution of methacryloyl chloride (11.6g, 111mmol) in anhydrous tetrahydrofuran (50mL) to obtain a reaction solution; after the reaction solution was raised to room temperature and continued to react for 5 hours, the reaction solution was concentrated under vacuum to remove the solvent, and added 50mL of e...
Embodiment 3
[0045] This embodiment provides a photoresist resin monomer synthesized by hexahydro-1H-indene-1,3(2H)-dione, and the reaction scheme of the synthesis method of the photoresist resin monomer is as follows:
[0046]
[0047] It specifically includes the following steps:
[0048] S1. Preparation of ethyl Grignard reagent: first add 3.2g of magnesium chips (132mmol) to 15ml of anhydrous ether, and add an iodine tablet to obtain a reaction solution; then, dissolve 14.4g of ethyl bromide (132mmol) in 25mL Prepare ethyl bromide ether solution in diethyl ether; then, under the protection of nitrogen, first add 6mL ethyl bromide ether solution to the above reaction solution. After 5 minutes, the reaction solution boils slightly, and the color of iodine disappears. Under stirring, continue to Add the remaining ethyl bromide ether solution dropwise, and add 20 mL of diethyl ether, raise the temperature to keep boiling slightly, and reflux for half an hour to obtain ethyl Grignard rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com