Method for improving iron-carbon micro-electrolysis reaction efficiency by utilizing iron-containing waste acid solution wastewater
A technology of iron-carbon micro-electrolysis and reaction efficiency, which is applied in the fields of metallurgical wastewater treatment, chemical instruments and methods, and textile industry wastewater treatment, etc. problem, to prevent passivation, enhance the treatment effect, and inhibit the effect of oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
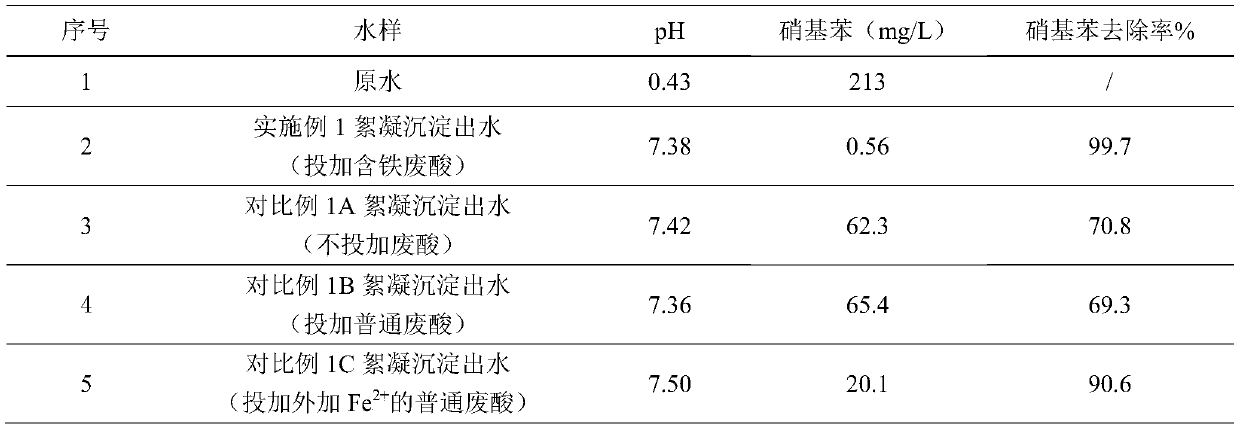
[0030] In this embodiment, the iron-carbon micro-electrolysis unit is mainly used to reduce nitrobenzene in wastewater. The wastewater comes from the red and yellow mixed wastewater in the nitrobenzene (TDI) production wastewater. The wastewater is strongly acidic and contains a large amount of nitrobenzene. Because nitrobenzene has a high redox potential, it will have a greater impact on anaerobic treatment. influences. Therefore, iron-carbon micro-electrolysis is generally used to reduce nitrobenzene in wastewater to aniline, and the reduced wastewater is biologically treated by anaerobic / hydrolytic acidification process to reduce the cost of wastewater treatment.
[0031]In this example, the red-yellow mixed wastewater in the TDI production wastewater was taken as the research object, and the nitrobenzene in the wastewater was reduced by iron-carbon micro-electrolysis. During the experiment, the continuous flow experiment was mainly carried out. The iron-carbon micro-elect...
Embodiment 2
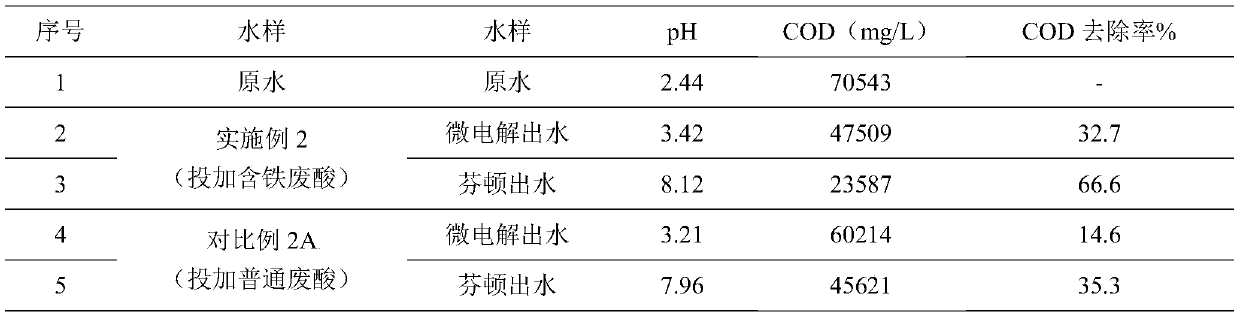
[0051] In this example, the wastewater from the pharmaceutical industry is taken as the research object. The organic matter in the wastewater is relatively high, and it is difficult to degrade the organic matter. It is necessary to carry out iron-carbon micro-electrolysis + Fenton coupling process to pretreat the wastewater. The raw water quality indicators of medical wastewater are shown in Table 2.
[0052] Table 2 Raw water indicators of medical wastewater
[0053]
[0054] It can be seen from the data in Table 2 that the pH of the raw water is 2.44, which is within the suitable pH range for iron-carbon micro-electrolysis, so the iron-containing waste acid solution was directly added in the experiment. The experimental process is as follows:
[0055] (1) Add iron-containing waste acid: Take 1L of waste water sample in a beaker, add 5ml of iron-containing waste acid solution, the iron-containing waste acid solution comes from pickling waste solution in the electroplating...
Embodiment 3
[0072] In this example, the wastewater from the printing and dyeing industry is taken as the research object. The wastewater has high chroma, high concentration of refractory organic matter, and poor biodegradability. Therefore, the main purpose of the study in this example is to use iron-carbon micro-electrolysis to remove the chroma of wastewater while improving the B / C of wastewater. The raw water quality indicators are shown in Table 4.
[0073] Table 4 Raw water indicators of printing and dyeing wastewater
[0074]
[0075] It can be seen from the data in Table 4 that the pH value of the raw water is 6.54, and the pH value can be directly adjusted by the iron-containing waste acid solution. The waste water has low B / C, poor biochemical properties, and the color is dark red.
[0076] The experimental process is as follows:
[0077] (1) Adjusting pH: Take 1L of wastewater water sample in a beaker, adjust the pH value of wastewater to 3.13 by using iron-containing wast...
PUM
| Property | Measurement | Unit |
|---|---|---|
| clearance rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com