Fluoreno-aza-naphthalene derivative, synthesis method and electronic device thereof
A technology for electronic devices and aziridines, which is applied in the field of electronic devices, fluorenazine derivatives and their synthesis, and achieves the effects of simple preparation methods, broad industrialization prospects, and reduced driving voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] Embodiment 1: the synthesis of compound 2-15
[0136] (Synthesis of intermediate M1)
[0137] The synthetic route of intermediate M1 is as follows:
[0138]
[0139] P-bromobenzohydrazide (4.3g, 20mmol), ninhydrin (3.6g, 20mmol), ammonium acetate (12.0g, 90mmol) and 120mL acetic acid were successively added into a 250mL single-necked flask, and the reaction was stirred under reflux for 12 hours. After the reaction was complete, the solid was collected by suction filtration and washed with a small amount of absolute ethanol. The crude product was further purified by column chromatography (petroleum ether:dichloromethane=3:1 (V / V)). The solvent was evaporated, and after drying, 3.9 g of a light yellow solid was obtained, with a yield of 58%. MS (EI): m / z: 336.58 [M + ]. Anal.calcdforC 16 h 8 BrN 3 O (%): C 56.83, H 2.38, N 12.43; found: C 56.75, H 2.35, N 12.40.
[0140] (Synthesis of Intermediate M2)
[0141] The synthetic route of intermediate M2 is as foll...
Embodiment 2
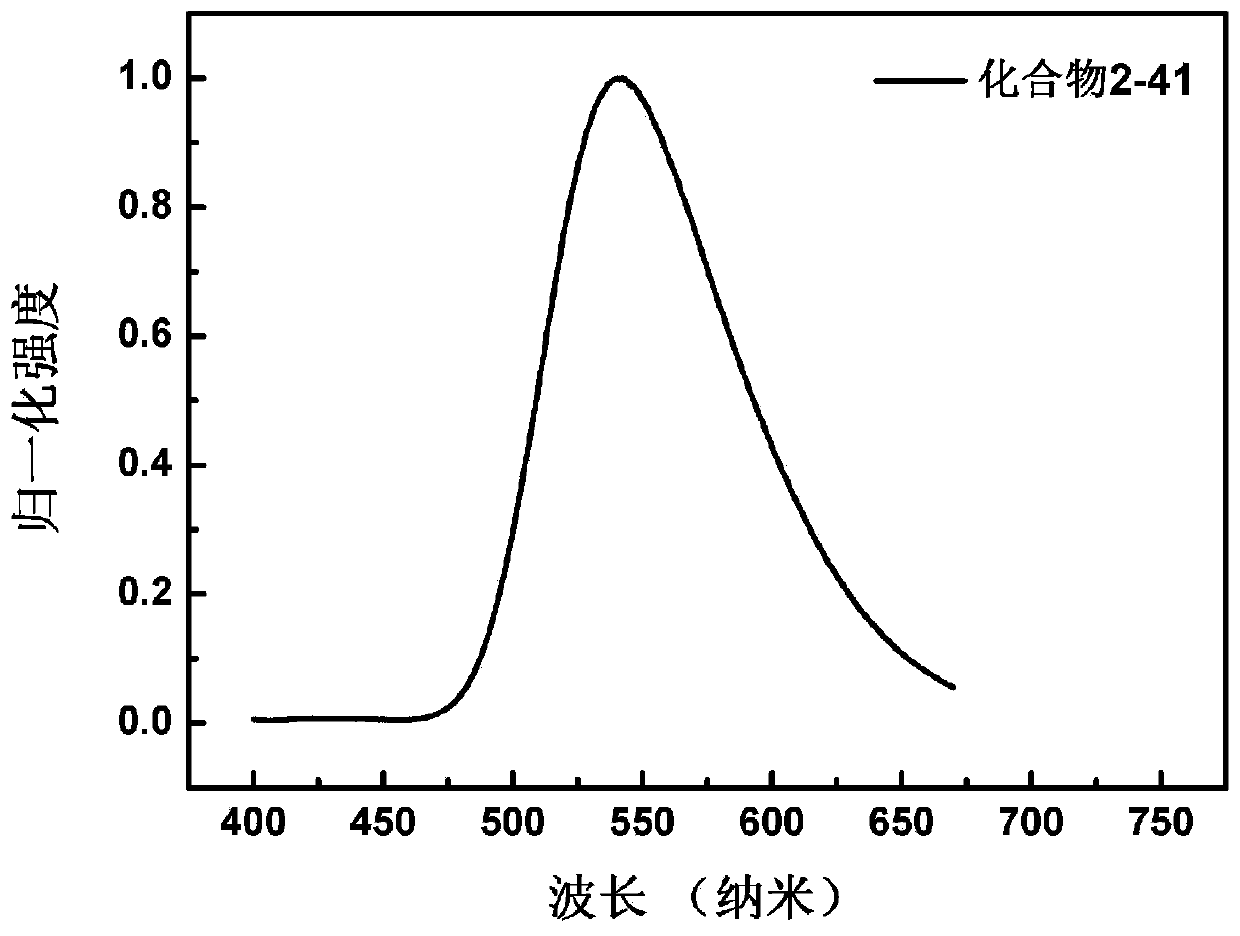
[0156] Embodiment 2: the synthesis of compound 2-41
[0157] (Synthesis of compound 2-41)
[0158] The synthetic route of compound 2-41 is as follows:
[0159]
[0160] In a 250mL two-necked flask, add 9.2g (23.0mmol) M4, 5.2g (29.0mmol) carbazole, 3.0g (29.0mmol) sodium tert-butoxide, 0.1g (0.3mmol) tri-tert-butylphosphine tetrafluoroborate, 0.27g (0.3mmol) of tris(dibenzylideneacetone)dipalladium, after degassing the reaction system, under the protection of nitrogen, add 150mL of toluene, stir and heat to reflux for 12 hours. After the reaction was complete, the system was cooled to room temperature, filtered under reduced pressure, and the filter residue was washed with a large amount of dichloromethane, and the filtrate was concentrated to obtain a crude product, which was eluted with petroleum ether:dichloromethane=2:3 (volume ratio) The reagent was separated and purified on a silica gel column to obtain 10.3 g of a white solid with a yield of 92%. MS (EI): m / z 488....
Embodiment 3
[0161] Embodiment 3: the synthesis of compound 2-1
[0162] (Synthesis of compound 2-1)
[0163] Compound 2-1 synthetic route is as follows:
[0164]
[0165] In a 250mL two-necked flask, add 9.2g (23.0mmol) M4, 5.2g (29.0mmol) diphenylamine, 3.0g (29.0mmol) sodium tert-butoxide, 0.1g (0.3mmol) tri-tert-butylphosphine tetrafluoroborate, 0.27g (0.3mmol) of tris(dibenzylideneacetone)dipalladium, after degassing the reaction system, under the protection of nitrogen, add 150mL of toluene, stir and heat to reflux for 12 hours. After the reaction was complete, the system was cooled to room temperature, filtered under reduced pressure, and the filter residue was washed with a large amount of dichloromethane, and the filtrate was concentrated to obtain a crude product, which was eluted with petroleum ether:dichloromethane=2:3 (volume ratio) The reagent was separated and purified on a silica gel column to obtain 10.3 g of a white solid with a yield of 92%. MS (EI): m / z 490.43 [M+...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com