Repairable cross-linked solid polymer electrolyte as well as preparation method and application thereof
A solid polymer and electrolyte technology, applied in non-aqueous electrolyte batteries, electrolyte immobilization/gelation, circuits, etc., can solve the problems of LMBs failure, easy fracture, leakage of chemical electrolyte, etc., and achieve good electrochemical performance , good thermal stability, good self-healing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0040] Weigh 0.0056g of terephthalaldehyde (TPA), 0.25g of polyethylene glycol diamine (NH 2 -PEG-NH 2 ), and 0.0284g of bisphenol A diglycidyl ether (DGEBA) was added to an appropriate amount of anhydrous acetonitrile solvent. Then the mixture was stirred on an intelligent constant temperature and timed magnetic stirrer for 4 hours to make it dissolve completely. Use a disposable plastic dropper to drop the homogeneous and transparent mixture obtained by stirring on the polytetrafluoroethylene mold. The solvent was evaporated at normal temperature (until the obtained liquid became a sol state). Finally, put it into a vacuum drying oven and heat it at 100°C for 6 hours to obtain a yellow transparent and brittle film.
Embodiment example 2
[0042] Weigh 0.0083g of terephthalaldehyde (TPA), 0.25g of polyethylene glycol diamine (NH 2 -PEG-NH 2 ) and 0.0426g of bisphenol A diglycidyl ether (DGEBA) were then added with 0.1466g of lithium bistrifluoromethanesulfonimide (LiTFSI), dissolved in acetonitrile solvent, and then the sample was stirred on an intelligent constant temperature timing magnetic stirrer 4h to dissolve completely. Use a disposable plastic dropper to drop the transparent mixture obtained by stirring onto the Teflon mold. Develop at room temperature, volatilize the solvent (until the liquid becomes a sol state), and finally heat-cure at 100°C for 8 hours, and dry in vacuum for 48 hours to obtain a yellow transparent flexible polymer film.
Embodiment example 3
[0044] Weigh 0.0042g of terephthalaldehyde (TPA), 0.25g of polyethylene glycol diamine (NH 2 -PEG-NH 2) and 0.0213g of bisphenol A diglycidyl ether (DGEBA) were then added with 0.1371g of lithium bistrifluoromethanesulfonylimide (LiTFSI), dissolved in acetonitrile solvent, and then the sample was stirred on an intelligent constant temperature timing magnetic stirrer 4h to dissolve completely. Use a disposable plastic dropper to drop the transparent mixture obtained by stirring onto the Teflon mold. Develop at room temperature, volatilize the solvent (until the liquid becomes a sol state), and finally heat-cure at 100°C for 12 hours, and dry in vacuum for 48 hours to obtain a yellow transparent flexible polymer film.
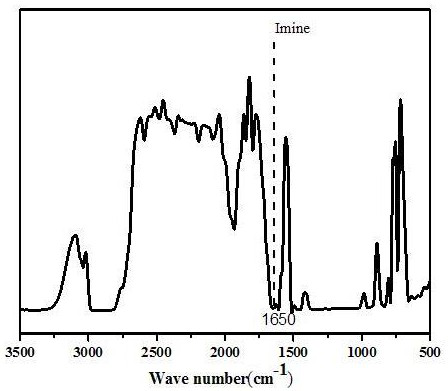
[0045] figure 1 It is the infrared spectrogram of the polymer electrolyte thin film that embodiment 3 prepares, from figure 1 It can be seen in: at 1650cm -1 A strong imine bond absorption band (-N=CH-) appeared at , indicating the formation of Schiff base b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com