Novel bridged tetradentate fourth-subgroup metal complex, and preparation method and application thereof
A metal complex, the fourth technology, which can be applied to the compounds of Group 4/14 elements of the periodic table, titanium organic compounds, chemical instruments and methods, etc., and can solve the problems of low catalytic efficiency, limited industrial application, and deactivation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
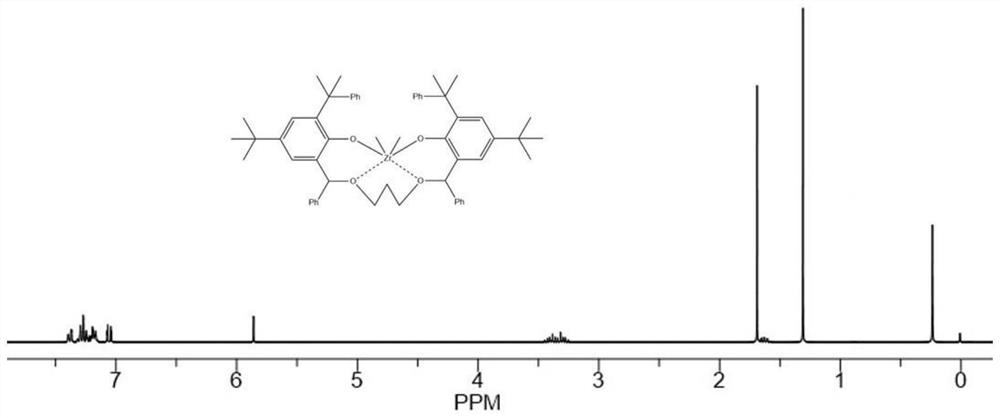
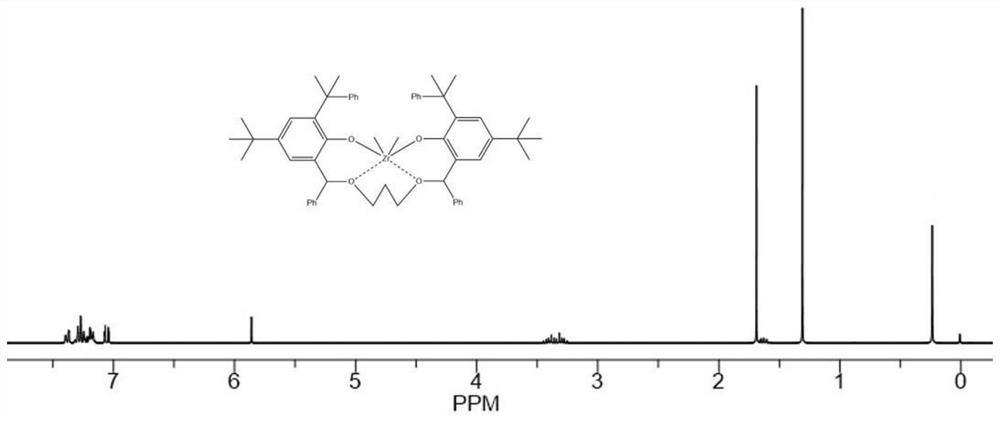
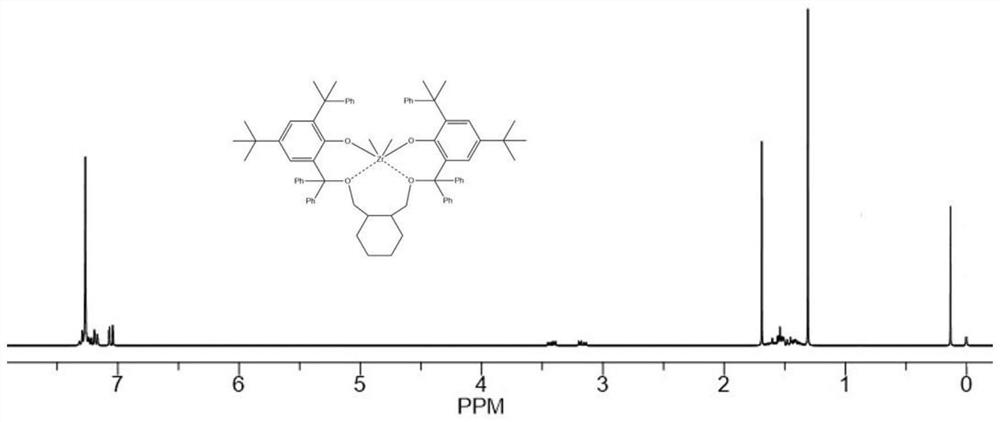
Image
Examples
preparation example Construction
[0183] The present invention provides a method for preparing the novel bridged tetradentate fourth subgroup metal complex described in the above technical solution, comprising the following steps:
[0184] will have a ligand of formula b and MX 4 reaction to obtain a novel bridging tetradentate fourth subgroup metal complex with a structure of formula a;
[0185]
[0186] In the present invention, the ligand having formula b is preferably prepared according to the following method:
[0187] The compound of formula I is brominated to obtain the compound of formula II;
[0188] The compound of the formula II is mixed with KOH and chloromethyl methyl ether (CH 3 OCH 2 Cl) reaction, obtains the compound of formula III;
[0189] reacting the compound of formula III with n-butyllithium, and then reacting with the compound of formula IV to obtain the compound of formula V;
[0190] Reacting the compound of the formula V structure with a dibromo compound to obtain a ligand hav...
Embodiment 1
[0224](1). Synthesis of related compounds containing bridging group Y with rigid ring structure
[0225] ①Synthesis of 1,2-dibromomethylcyclohexane
[0226]
[0227] Under nitrogen atmosphere, will contain LiAlH 4 (300mmol) in tetrahydrofuran (200mL) was cooled to 0°C, and the tetrahydrofuran (150mL) solution containing 1,2-cyclohexanedicarboxylic acid (150mmol) was added dropwise into the above system. , then reflux for 12h, stop the reaction, cool to 0°C, carefully add ammonium chloride powder thereinto, stop when no gas is produced in the reaction flask, add 15% sodium sulfate solution (150mL), then add diethyl ether (150mL), The organic phase was separated and retained, and the aqueous phase was extracted three times with diethyl ether. The organic phases were combined, dried over anhydrous magnesium sulfate, filtered, and the solvent was removed by rotary evaporation. The obtained crude product was frozen and recrystallized in methanol (-40°C) to obtain 1, 2-Dihydrox...
Embodiment 2
[0251] On the basis of Example 1, the complexes of P1~P36 structures were further prepared:
[0252] P1:R 1 = methyl, R 2 = methyl, R 3 = Hydrogen, R 4 =phenyl, Y=carbon three straight chains, M=Zr, X=methyl;
[0253] P2:R 1 = tert-butyl, R 2 = tert-butyl, R 3 =hydrogen, R4=phenyl, Y=carbon three straight chains, M=Zr, X=methyl;
[0254] P3:R 1 =3,5-di-tert-butylphenyl, R 2 = tert-butyl, R 3 = Hydrogen, R 4 =phenyl, Y=carbon three straight chains, M=Zr, X=methyl;
[0255] P4:R 1 = 1-naphthyl, R 2 = tert-butyl, R 3 = Hydrogen, R 4 =phenyl, Y=carbon three straight chains, M=Zr, X=methyl;
[0256] P5:R 1 =9-anthracenyl, R 2 = tert-butyl, R 3 = Hydrogen, R 4 =phenyl, Y=carbon three straight chains, M=Zr, X=methyl;
[0257] P6:R 1 = carbazolyl, R 2 = tert-butyl, R 3 = Hydrogen, R 4 =phenyl, Y=carbon three straight chains, M=Zr, X=methyl;
[0258] P7:R 1 = cumyl, R 2 = tert-butyl, R 3 = Hydrogen, R 4 =phenyl, Y=carbon three straight chains, M=Zr, X=meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com