Perylene diimide electron transport material, synthesis method and application thereof
An electron transport material, perylene diimide technology, applied in circuits, photovoltaic power generation, electrical components, etc., can solve problems such as unfavorable charge extraction and transmission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
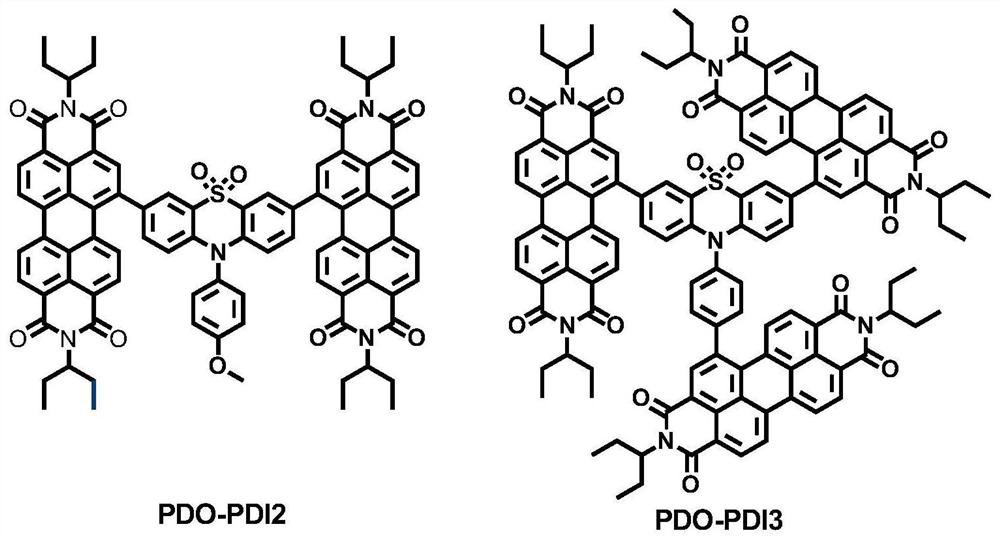
[0056] Synthesis of electron transport material PDO-PDI2 and its application in trans-planar perovskite solar cells:
[0057]
[0058] Compound 1: Add p-methoxybromobenzene (0.823g, 4.40mmol), phenothiazine (0.797 g, 4.00mmol), catalyst palladium acetate (0.018g, 0.08mmol), tert-butanol in a dry round bottom flask Sodium (0.576g, 6.00mmol) and solvent toluene (80mL), under nitrogen protection, stirred at room temperature for 15min, added tri-tert-butylphosphine (0.1g, 0.05mmol), heated to 110°C for 12h. Stop heating, cool to room temperature, add 150mL distilled water and 50mL dichloromethane to the reaction solution for extraction, collect the organic layer, repeat three times, remove the solvent under reduced pressure, the mixture is separated and purified by silica gel chromatography, and the eluent is petroleum ether / dichloromethane (5:1 vol / vol), dried in vacuo to obtain compound 1 (0.991 g, yield: 81.16%). 1 H-NMR (400MHz, CDCl 3 ), δ (ppm): 7.34 (d, J = 8.7Hz, 2H)...
Embodiment 2
[0066] Synthesis of electron transport material PDO-PDI3 and its application in trans-planar perovskite solar cells:
[0067]
[0068] Compound 1: Add bromobenzene (0.686g, 4.40mmol), phenothiazine (0.797g, 4.00mmol), catalyst palladium acetate (0.018g, 0.08mmol), sodium tert-butoxide (0.576g , 6.00mmol) and solvent toluene (60 mL), under nitrogen protection, stirred at room temperature for 15min, added tri-tert-butylphosphine (0.1g, 0.05mmol), heated to 110°C for 12-14h. Stop heating and cool to room temperature, add 150mL distilled water and 50mL dichloromethane to the reaction solution for extraction, collect the organic layer, repeat three times, remove the solvent under reduced pressure, and the mixture is separated and purified by silica gel column chromatography, the eluent is petroleum ether / dichloromethane Chloromethane (5:1 vol / vol), dried in vacuo to obtain compound 1 (0.893 g, yield: 81.16%). 1 H NMR (400MHz, CDCl3) δ7.62(d, J=16.8Hz, 2H), 7.47(t, J=7.4Hz, 1H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com