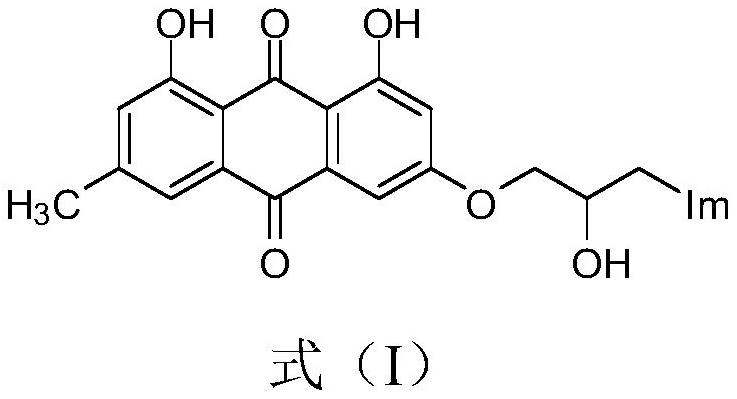
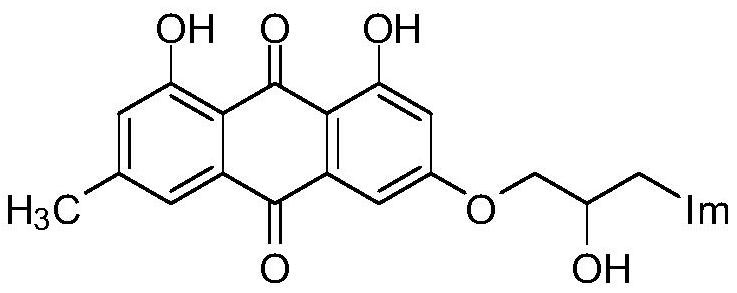
Emodin azole alcohol compound and application thereof
A technology of emodinazole and compounds, applied in organic chemistry, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problems of unsatisfactory antimicrobial effect, achieve strong in vitro antimicrobial activity, and simple process , low-cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Prepare emodinazole compound I-1, the reaction formula is as follows:
[0033]
[0034] Specific steps are as follows:
[0035] Add 0.101g of 1,8-dihydroxy-3-methyl-6-(oxirane-2-ylmethoxy)anthracene-9,10-dione (shown in formula II) in the 50mL round bottom flask ), 0.053g of potassium carbonate, 0.022g of 1,2,4-triazole, and 30mL of absolute ethanol were refluxed for reaction, tracked by thin-layer chromatography until the end of the reaction, cooled to room temperature (18~25°C), and ethanol was removed by distillation under reduced pressure , the residue was purified by silica gel column chromatography using a mixture of dichloromethane and methanol at a volume ratio of 10:1 as the eluent, and dried to obtain 0.151 g of emodinazole compound I-1 as a yellow solid.
[0036] The yield of this embodiment is 64%; melting point 124-126 ℃; 1 H NMR (400MHz, DMSO-d 6 )δppm: 2.35(s,3H,CH 3 ),4.07-4.03(m,H,OCH 2 CHCH 2 ), 4.14-4.11 (m, H, OCH 2 CHCH 2 ), 4.14-4.11 (m,...
Embodiment 2
[0039] Emodin azole compound I-2, the reaction formula is as follows:
[0040]
[0041] Specific steps are as follows:
[0042] In the 50mL round bottom flask, add 0.149g of 1,8-dihydroxy-3-methyl-6-(oxirane-2-ylmethoxy)anthracene-9,10-dione (shown in formula II ), potassium carbonate 0.089g, 4-nitroimidazole 0.054g and absolute ethanol 30mL, reflux to react, thin-layer chromatography traces to the end of the reaction, cools to room temperature (18~25 ℃), removes ethanol by vacuum distillation, and the residue Purify by silica gel column chromatography using a mixture of dichloromethane and methanol at a volume ratio of 10:1 as the eluent, and dry to obtain 0.098 g of emodinazole compound I-2 as a yellow solid.
[0043] The yield of this example is 51%; melting point is 167-169°C; 1 H NMR (400MHz, DMSO-d 6 )δppm: 2.37(s,3H,CH 3 ),4.18-4.08(m,4H,OCH 2 CHCH 2 ),4.37-4.30(m,H,OCH 2 CHCH 2 ), 5.70 (s, H, OH), 6.83 (s, H, emodin-2-H), 7.10 (br, 2H, emodin-4, 5-H), 7.41 (...
Embodiment 3
[0045] Emodin azole compound I-3, the reaction formula is as follows:
[0046]
[0047] Specific steps are as follows:
[0048]In the 50mL round bottom flask, add 0.152g of 1,8-dihydroxy-3-methyl-6-(oxirane-2-ylmethoxy)anthracene-9,10-dione (shown in formula II ), potassium carbonate 0.091g, 2-methyl-5-nitroimidazole 0.061g and absolute ethanol 30mL, reflux for reaction, TLC tracking to the end of the reaction, cool to room temperature (18~25°C), and distill under reduced pressure Remove ethanol, the residue is purified by silica gel column chromatography with a mixture of dichloromethane and methanol at a volume ratio of 10:1 as an eluent, and dried to obtain 0.087g of yellow solid-like emodinazole compound I-3 .
[0049] The yield of this example is 42%; the melting point is 145-147°C; 1 H NMR (400MHz, DMSO-d 6 )δppm: 22.37(s,3H,CH 3 ),2.39(s,3H,imidazole-CH 3 ),4.21-4.10(m,5H,OCH 2 CHCH 2 ),5.66(br,H,OH),8.27(s,H,imidazole-4-H),8.38(s,H,imidazole-3-H),11.91(s,H,e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com