Preparation method of redox type gel electrolyte for all-solid-state supercapacitor
A technology of supercapacitor and gel electrolyte, applied in the direction of hybrid capacitor electrolyte, etc., can solve the problems of poor cycle performance, unusable production, obvious self-discharge effect, etc., to improve energy storage density, ensure high-rate charge and discharge, and improve Effects of Cycling Stability and Self-Discharge Performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
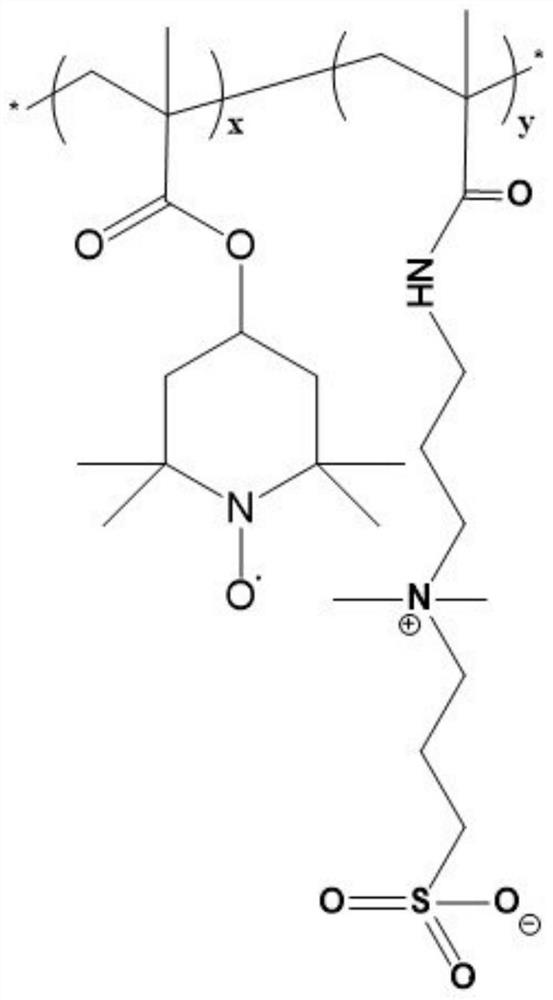
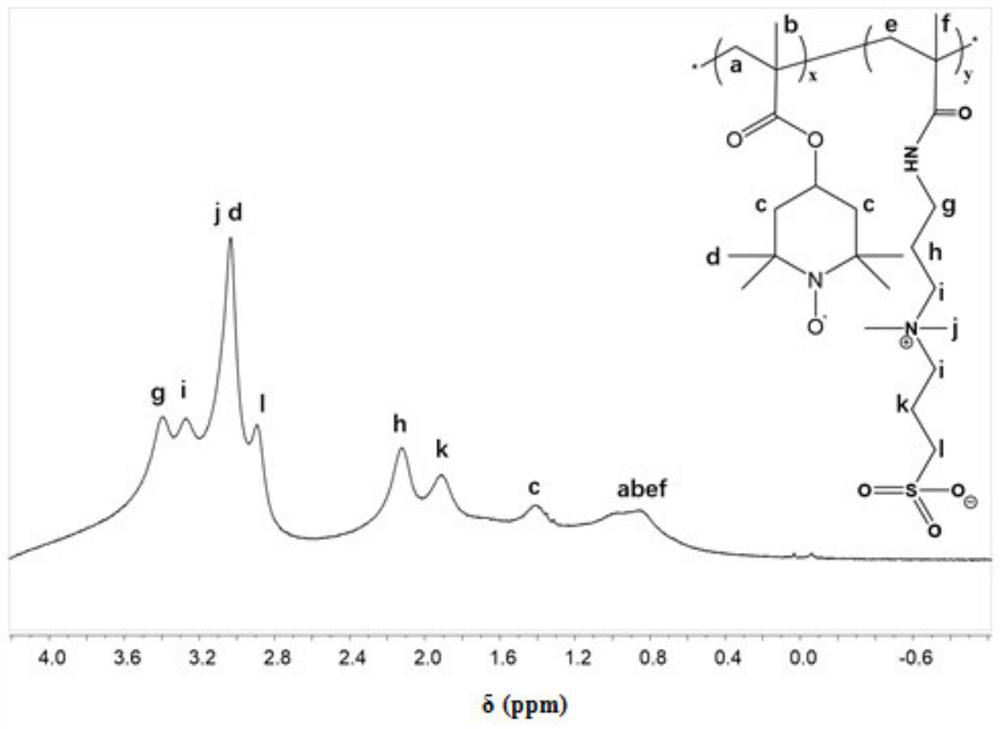
[0035] A redox gel electrolyte (P(PDP-co-TEMPO)) with high ionic conductivity and high specific energy, which is a copolymer of an amphoteric monomer and a stable redox monomer substance, its structure is shown in figure 1 .
[0036] Further, the preparation method of P(PDP-co-TEMPO) comprises the following steps:
[0037] Step (1) Synthesis of P(PDP-co-TEMPO) copolymer: add 1.0g amphoteric monomer [3-(methacrylamido) propyl group] dimethyl (3-thio Propyl)ammonium hydroxide inner salt) and 9.0mg redox active monomer 2-methyl-2-acrylic acid-2,2,6,6-tetramethyl-4-piperidinyl ester and 4.0ml to Ionized water, fully stirred and dissolved, and then added 1.0 mg of 4,4'-azo (4-cyanovaleric acid) as an initiator. Freeze the reaction system with liquid nitrogen, then use a vacuum pump for 12 minutes, repeat the above steps three times, keep the system anaerobic and control the temperature at 70°C, and react for 18 hours to obtain a copolymer precursor.
[0038] Step (2) Oxidation of...
Embodiment 2
[0044] Step (1) Synthesis of P(PDP-co-TEMPO) copolymer: add 0.5g amphoteric monomer [3-(methacrylamido) propyl group] dimethyl(3-thio Propyl)ammonium hydroxide inner salt) and 4.5mg redox active monomer 2-methyl-2-acrylic acid-2,2,6,6-tetramethyl-4-piperidinyl ester and 2.0ml to Ionized water, fully stirred and dissolved, and then added 0.5 mg of 4,4'-azo (4-cyanovaleric acid) as an initiator. Freeze the reaction system with liquid nitrogen, then use a vacuum pump for 10 minutes, repeat the above steps three times, keep the system anaerobic and control the temperature at 60°C, and react for 12 hours to obtain a copolymer precursor.
[0045] Step (2) Oxidation of the precursor: add an appropriate amount of sodium hydroxide to the precursor solution obtained in the previous step to neutralize the solution, then add 50 mg of sodium tungstate and 6 mg of hydrogen peroxide, and react for 36 hours to obtain an oxidized The reducing active nitrogen-oxygen free radical copolymer is P...
Embodiment 3
[0050] Synthesis of step (1) P (PDP-co-TEMPO) copolymer: (1) Synthesis of P (PDP-co-TEMPO) copolymer: in the hydrochloric acid solution of 0.75M, add 2.0g amphoteric monomer [3-( Methacrylamido) propyl] dimethyl (3-thiopropyl) ammonium hydroxide inner salt) and 18.0 mg redox active monomer 2-methyl-2-acrylic acid-2,2,6, 6-Tetramethyl-4-piperidinyl ester and 8.0ml of deionized water were fully stirred and dissolved, and then 2.0mg of 4,4'-azo (4-cyanovaleric acid) was added as an initiator. Freeze the reaction system with liquid nitrogen, then use a vacuum pump for 15 minutes, repeat the above steps three times, keep the system anaerobic and control the temperature at 80°C, and react for 24 hours to obtain a copolymer precursor.
[0051] Step (2) Oxidation of the precursor: add an appropriate amount of sodium hydroxide to the precursor solution obtained in the previous step to neutralize the solution, and then add 0.2g sodium tungstate and 24mg hydrogen peroxide to react for 48...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com