Emodin azole alcohol compound and preparation method thereof
A technology for emodinazole and compound, which is applied in the field of emodinol compound and its preparation, can solve problems such as unsatisfactory anti-microbial effect, achieve strong in vitro anti-microbial activity, solve drug resistance and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
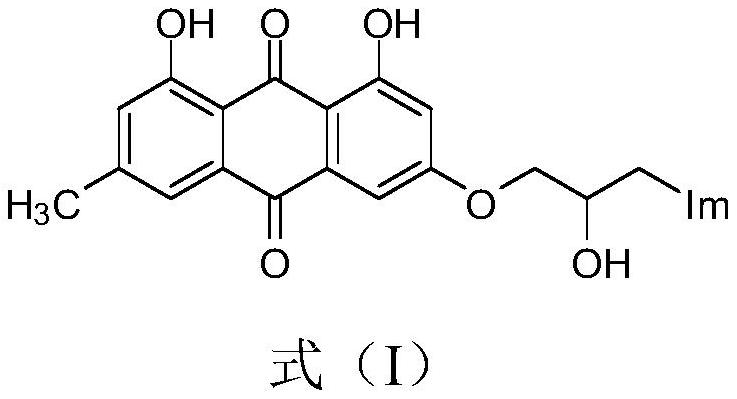
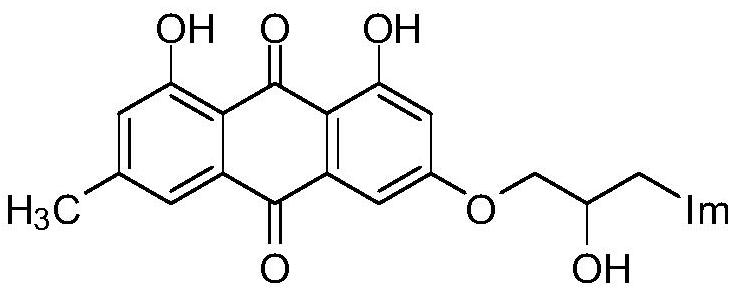
[0026] Prepare emodinazole compound I-1, the reaction formula is as follows:
[0027]
[0028] Specific steps are as follows:
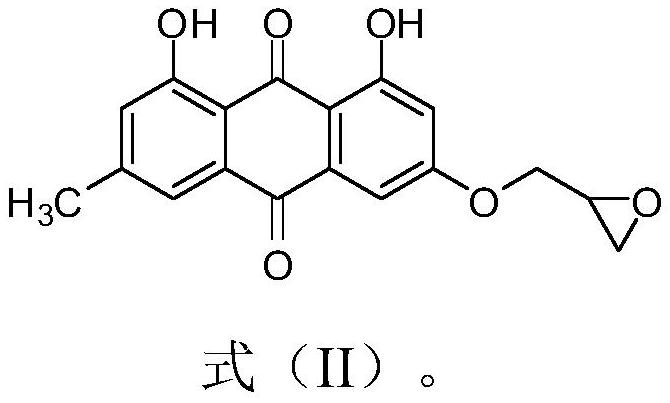
[0029] Add 0.101g of 1,8-dihydroxy-3-methyl-6-(oxirane-2-ylmethoxy)anthracene-9,10-dione (shown in formula II) in the 50mL round bottom flask ), 0.053g of potassium carbonate, 0.022g of 1,2,4-triazole, and 30mL of absolute ethanol were refluxed for reaction, tracked by thin-layer chromatography until the end of the reaction, cooled to room temperature (18~25°C), and ethanol was removed by distillation under reduced pressure , the residue was purified by silica gel column chromatography using a mixture of dichloromethane and methanol at a volume ratio of 10:1 as the eluent, and dried to obtain 0.151 g of emodinazole compound I-1 as a yellow solid.
[0030] The yield of this embodiment is 64%; melting point 124-126 ℃; 1 H NMR (400MHz, DMSO-d 6 )δppm: 2.35(s,3H,CH 3 ),4.07-4.03(m,H,OCH 2 CHCH 2 ), 4.14-4.11 (m, H, OCH 2 CHCH 2 ), 4.14-4.11 (m,...
Embodiment 2
[0033] Emodin azole compound I-2, the reaction formula is as follows:
[0034]
[0035] Specific steps are as follows:
[0036] In the 50mL round bottom flask, add 0.149g of 1,8-dihydroxy-3-methyl-6-(oxirane-2-ylmethoxy)anthracene-9,10-dione (shown in formula II ), potassium carbonate 0.089g, 4-nitroimidazole 0.054g and absolute ethanol 30mL, reflux to react, thin-layer chromatography traces to the end of the reaction, cools to room temperature (18~25 ℃), removes ethanol by vacuum distillation, and the residue Purify by silica gel column chromatography using a mixture of dichloromethane and methanol at a volume ratio of 10:1 as the eluent, and dry to obtain 0.098 g of emodinazole compound I-2 as a yellow solid.
[0037] The yield of this example is 51%; melting point is 167-169°C; 1 H NMR (400MHz, DMSO-d 6 )δppm: 2.37(s,3H,CH 3 ),4.18-4.08(m,4H,OCH 2 CHCH 2 ),4.37-4.30(m,H,OCH 2 CHCH 2 ), 5.70 (s, H, OH), 6.83 (s, H, emodin-2-H), 7.10 (br, 2H, emodin-4, 5-H), 7.41 (...
Embodiment 3
[0039] Emodin azole compound I-3, the reaction formula is as follows:
[0040]
[0041] Specific steps are as follows:
[0042] In the 50mL round bottom flask, add 0.152g of 1,8-dihydroxy-3-methyl-6-(oxirane-2-ylmethoxy)anthracene-9,10-dione (shown in formula II ), potassium carbonate 0.091g, 2-methyl-5-nitroimidazole 0.061g and absolute ethanol 30mL, reflux for reaction, TLC tracking to the end of the reaction, cool to room temperature (18~25°C), and distill under reduced pressure Remove ethanol, the residue is purified by silica gel column chromatography with a mixture of dichloromethane and methanol at a volume ratio of 10:1 as an eluent, and dried to obtain 0.087g of yellow solid-like emodinazole compound I-3 .
[0043] The yield of this example is 42%; the melting point is 145-147°C; 1 H NMR (400MHz, DMSO-d 6 )δppm: 22.37(s,3H,CH 3 ),2.39(s,3H,imidazole-CH 3 ),4.21-4.10(m,5H,OCH 2 CHCH 2),5.66(br,H,OH),8.27(s,H,imidazole-4-H),8.38(s,H,imidazole-3-H),11.91(s,H,e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com