Preparation method of 4-octylphenethyl alcohol
A technology of octylphenylethyl alcohol and phenylethyl alcohol, which is applied in the field of preparation of 4-octylphenylethyl alcohol, can solve the problems of high synthesis reaction temperature, unfavorable industrial production, waste water and waste gas treatment, etc., achieve low synthesis reaction temperature and realize greenization The effect of less production and by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A preparation method for 4-octylphenethyl alcohol, comprising the following steps:
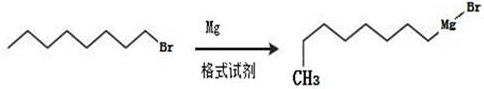
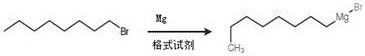
[0048] In the first step, the Grignard reagent adopts n-octyl magnesium bromide, and the synthesis of n-octyl magnesium bromide: bromooctane and magnesium powder are prepared by reacting in anhydrous ether or tetrahydrofuran (THF),
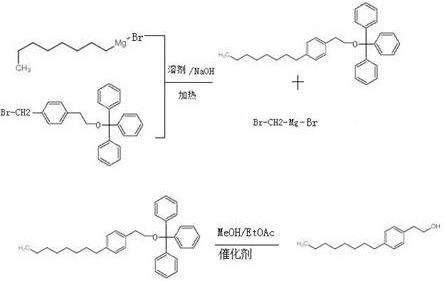
[0049] In the second step, p-methylbromphenethyl alcohol is alkylated with n-octyl magnesium bromide to obtain 4-octyl phenethyl alcohol and by-products.
[0050] The specific process is as follows:
[0051]
[0052] The present invention: R2-CH2-X2+R1-Mg-X1----R2-CH2-R1+X1-Mg-X2
[0053] Wherein: R1 is octyl, X1 is Br, R2 is phenethyl alcohol, X2 is Br.
[0054] In the present invention, in the first step, the solvent is one of THF and pyridine. Among them, THF is used as a solvent for the Grignard reagent reaction, which can increase the activity of the reaction between haloalkane and magnesium, and THF is a cyclic ether, so it is easier to complex ...
Embodiment 2
[0059] 1, the preparation of n-octylmagnesium bromide,
[0060] In a 500ml three-necked round-bottomed flask (drying treatment, connected to a desiccator), magnesium chips (1.5g, 61.3mmol) and a single iodine (catalyst) were added under nitrogen protection. After the iodine was heated and sublimated, 200ml of anhydrous ether was added, and then Slowly add bromooctane four times, after adding all the bromooctane, weakly reflux for 1 hour, the system is a gray and nearly transparent liquid, then stand still and suck out the supernatant; rotary evaporation removes anhydrous ether to obtain n-octyl base magnesium bromide salt. Then it was extracted with ethyl acetate to purify the n-octylmagnesium bromide salt.
[0061] 2, the preparation of 4-octylphenylethanol
[0062] In a 500mL round-bottomed flask, add tetrahydrofuran (200mL), add 50ml of sodium hydroxide solution, then add p-methylbromphenethyl alcohol, then add the n-octylmagnesium bromide salt obtained in the first step,...
Embodiment 3
[0064] 1, the preparation of n-octylmagnesium bromide,
[0065] In a 500ml three-necked round-bottomed flask (drying treatment, connected to a desiccator), magnesium chips (1.5g, 61.3mmol) and a single iodine (catalyst) were added under nitrogen protection. After the iodine was heated and sublimated, 200ml of anhydrous ether was added, and then Slowly add bromooctane four times, after adding all the bromooctane, weakly reflux for 1 hour, the system is a gray and nearly transparent liquid, then stand still and suck out the supernatant; rotary evaporation removes anhydrous ether to obtain n-octyl base magnesium bromide salt. Then it was extracted with ethyl acetate to purify the n-octylmagnesium bromide salt.
[0066] 2, the preparation of 4-octylphenylethanol
[0067] In a 500mL round-bottomed flask, add ethanol (200mL), add 50ml of sodium hydroxide solution, then add p-methyl bromide phenethyl alcohol, then add the n-octylmagnesium bromide salt obtained in the first step, he...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com