Anatase type TiO2 nanocrystalline with co-exposed {101}, {100} and {111}-crystal faces
A {101}, anatase-type technology, applied in the field of preparation of anatase-type TiO2 nanocrystals, can solve the problems of high price of the stripping reagent tetramethylammonium hydroxide, difficulty in large-scale industrial production, and difficulty in controlling the price , to achieve the effect of low synthesis cost, short production cycle and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Preparation of titanate sol: Measure 200mL of absolute ethanol in a round-bottomed flask with a graduated cylinder, place the above-mentioned round-bottomed flask in a water bath containing a mixture of ice and water, add 50mL of tetratitanate dropwise under stirring Isopropyl ester (0.166mol), after mixing and mixing, a transparent titanic acid sol is obtained;
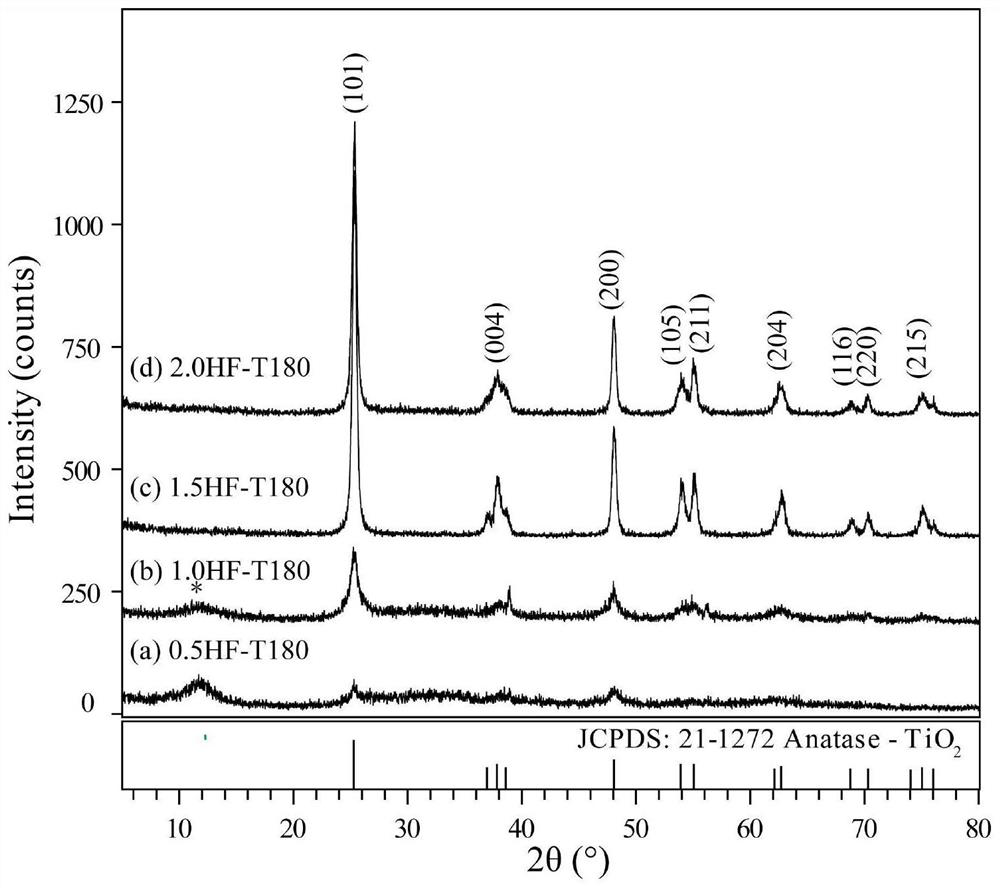
[0034] (2) Preparation of titanium dioxide gel: Take 60 mL of the titanic acid sol prepared in step (1) and transfer it to a hydrothermal reaction kettle, add 0.5 to 2.0 mL of HF solution (0.011 to 0.045 mol) dropwise under stirring conditions, and mix After mixing, put it in a constant temperature blast drying oven with a set temperature of 180°C, conduct a solvothermal reaction for 24 hours, take it out after cooling to room temperature, and filter the obtained product with a multi-purpose vacuum pump of a circulating water type, and wash it with a large amount of distilled water until the filtrate until...
Embodiment 2
[0046] (1) Preparation of titanate sol: Measure 100mL of absolute ethanol in a round-bottomed flask with a graduated cylinder, place the above-mentioned round-bottomed flask in a water bath containing a mixture of ice and water, add 25mL of tetratitanate dropwise under stirring Isopropyl ester, after mixing and mixing, a transparent titanic acid sol is obtained;
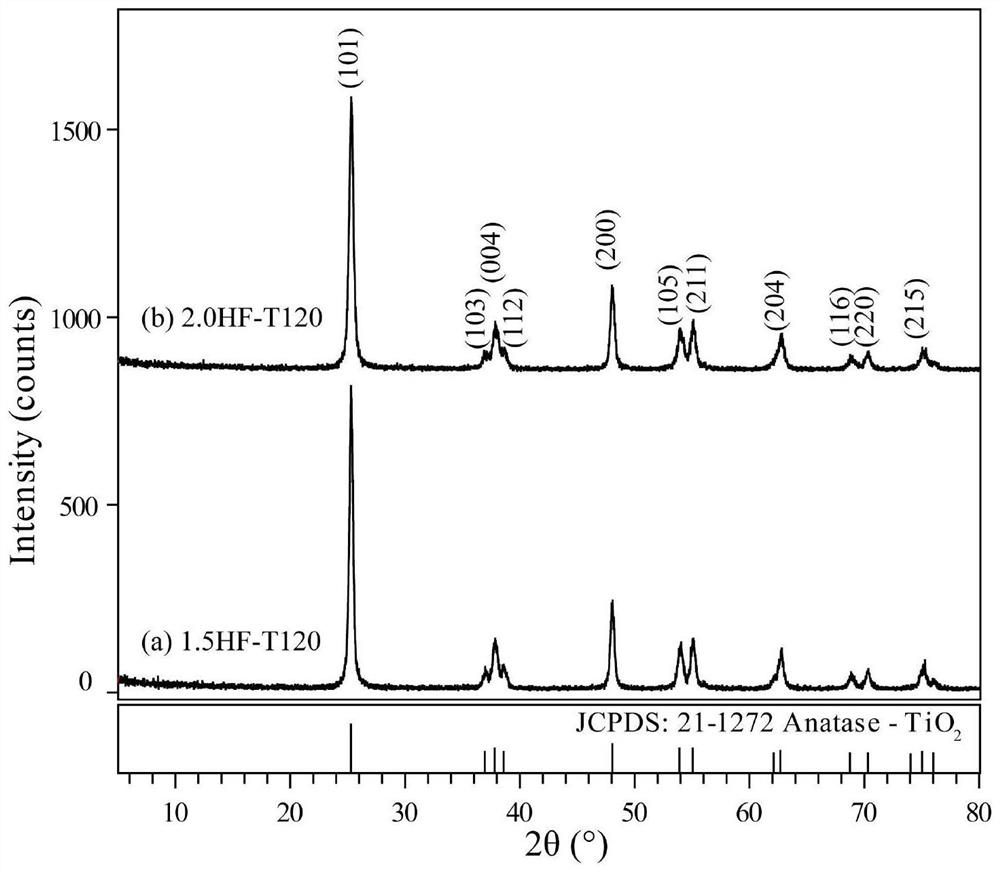
[0047] (2) Preparation of titanium dioxide gel: Take 60 mL of titanic acid sol prepared in step (1) and transfer it to a hydrothermal reaction kettle, and add 1.5 mL (0.034 mol) and 2.0 mL (0.045 mol) of HF dropwise under stirring conditions After mixing the solution, put it in a constant temperature blast drying oven with a set temperature of 120°C, react with solvent heat for 6 hours, take it out after cooling to room temperature, and filter the obtained product with a circulating water type multi-purpose vacuum pump, and wash it with a large amount of distilled water , until the filtrate is neutral; then place the...
Embodiment 3
[0052] (1) Preparation of titanate sol: Measure 210mL of absolute ethanol in a round-bottomed flask with a graduated cylinder, place the above-mentioned round-bottomed flask in a water bath containing a mixture of ice and water, add 60mL of tetratitanate dropwise under stirring Isopropyl ester (0.199mol), after mixing and mixing, a transparent titanic acid sol is obtained;
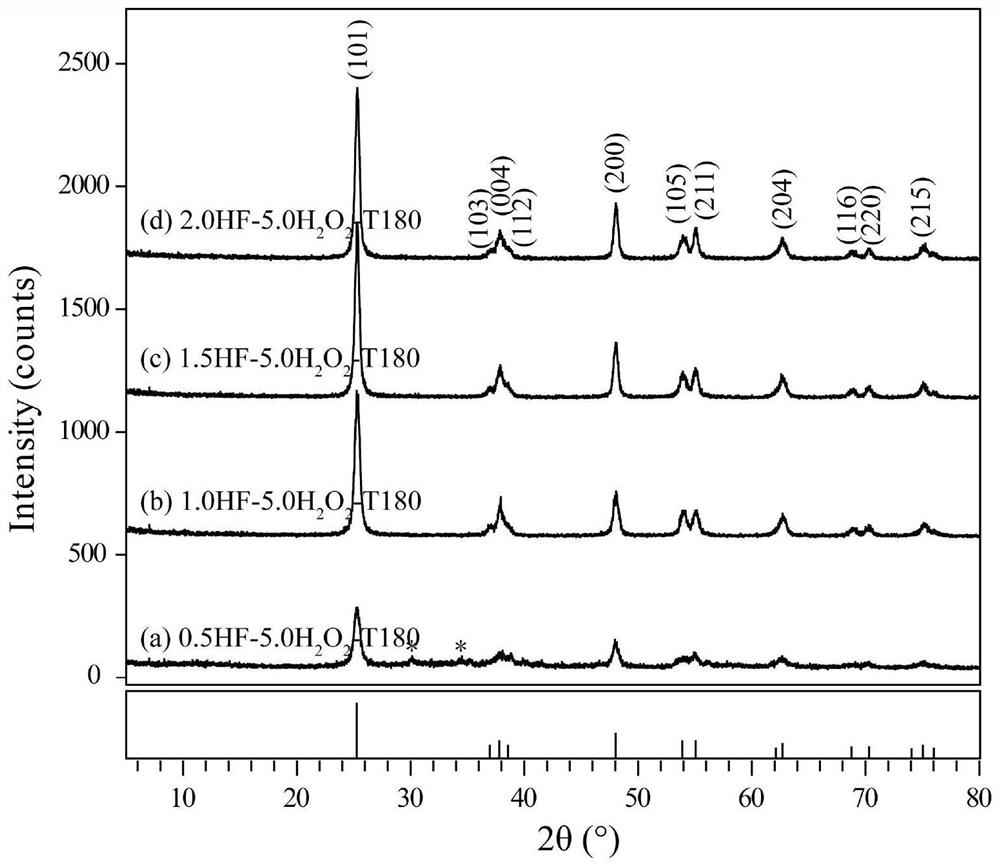
[0053] (2) Preparation of titanium dioxide gel: Take 60 mL of the titanic acid sol prepared in step (1) and transfer it to a hydrothermal reaction kettle, add 0.5 to 2.0 mL of HF solution (0.011 to 0.045 mol) dropwise under stirring conditions, and mix After mixing, slowly add 5.0 mL H 2 o 2 Solution (0.049mol), after being stirred evenly, was placed in a constant temperature blast drying oven with a set temperature of 180°C, solvothermal reaction was carried out for 24 hours, and it was taken out after cooling to room temperature. And wash with a large amount of distilled water until the filtrate is neu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com