Preparation method and application of falecalcitriol intermediate
A technology for an intermediate, calcidol, applied in the field of preparation of fluorocalcidol intermediates, can solve the problems of high cost, low safety and yield, expensive raw materials, etc., and achieves simplified reaction steps, reduced cost, and cost of raw materials. Inexpensive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
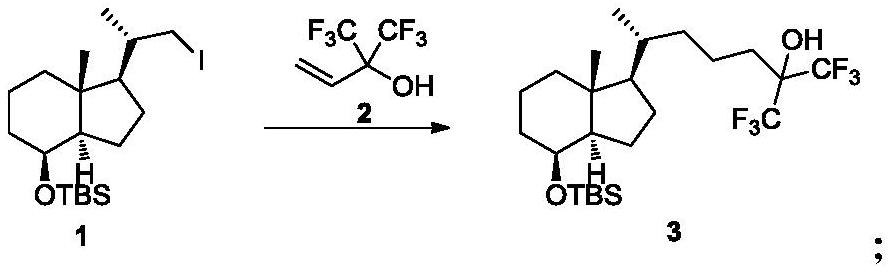
[0046] Embodiment 1: the preparation of compound 3
[0047] Add nickel chloride hexahydrate (81.8g, 343.9mmol) and zinc powder (22.4g, 343.9mmol) into a 500ml three-necked flask, add compound 2 (133.4g, 687.8mmol) and 200.0ml pyridine to the reaction system, and start stirring , the reaction system was green and turbid, replaced with inert gas three times, heated the reaction to 60°C-65°C for 30 minutes, stopped heating and lowered the temperature to 20°C-25°C, kept the temperature and used 100ml of compound 1 (30g, 68.8mmol) Pyridine was dissolved and added dropwise to the above reaction system, kept the temperature for 1.5h, filtered through diatomaceous earth, washed the filter cake with ethyl acetate, washed the organic phase with dilute hydrochloric acid, washed with saturated saline, dried, concentrated and passed through silica gel 15.6 g of compound 3 was obtained by column chromatography, with a yield of 45.0%.
Embodiment 2
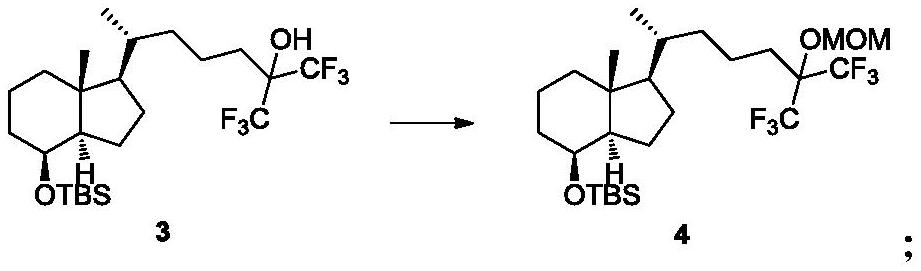
[0048] Embodiment 2: the preparation of compound 4
[0049] Under the protection of argon, add compound 3 (5.0g, 9.9mmol) to a 100mL three-necked flask and dissolve it in 760.0ml of dichloromethane, add diisopropylethylamine (6.4g, 49.5mmol), cool down to 0°C and add Methoxychloromethane (4.0g, 49.5mmol), naturally warmed to room temperature, stirred for 48 hours, the reaction solution was added to aqueous sodium bicarbonate solution, extracted with ethyl acetate, dried and concentrated to obtain the crude product, which was obtained by silica gel column chromatography 3.8 g of compound 4, yield 70.0%.
Embodiment 3
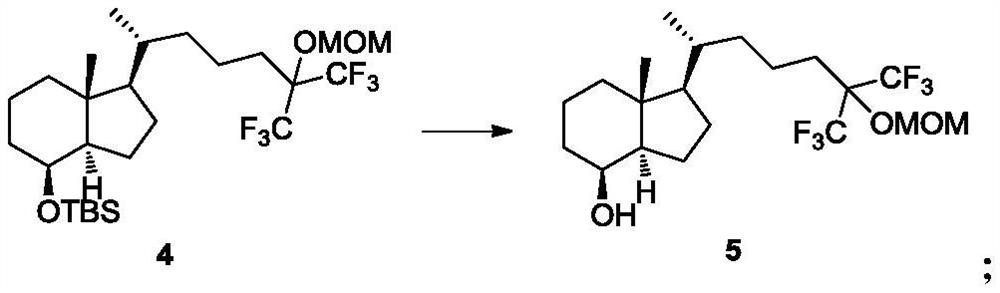
[0050] Embodiment 3: the preparation of compound 5
[0051]Compound 4 (8.0g, 14.6mmol) was added to a 250mL three-necked flask, 150.0ml (1854mmol) of tetrahydrofuran was added, a tetrahydrofuran solution of tetrabutylammonium fluoride trihydrate (23.0g, 72.9mmol) was added, and heated to reflux for 50 hours. Saturated aqueous sodium bicarbonate was added, extracted with ethyl acetate, dried and concentrated to obtain a crude product, which was subjected to silica gel column chromatography to obtain 4.6 g of compound 5 with a yield of 72.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com