Cathode catalyst active material for hydrogen fuel cell, preparation method and catalyst
A cathode catalyst and fuel cell technology, applied in battery electrodes, nanotechnology for materials and surface science, circuits, etc., to achieve the effects of improving activity, increasing surface area, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Weigh 20.0mg Pt(acac) 2 , 10.0mg Ni(acac) 2 , 11.0mg Ga(acac) 3 , 2.4mg NH 4 ReO 4 , 75.0 mg of CTAB was poured into the reaction bottle, 4.0 mL of oleylamine was added thereto, and then ultrasonicated in an ultrasonic machine for 30 min to form a homogeneous system. Then weigh 20.0mg W(CO) 6 Add it into the homogeneous system formed after ultrasonication, tighten the bottle cap, and turn the reaction bottle to make W(CO) 6 Spread relatively evenly on the bottom of the reaction bottle, and finally transfer to an oil bath at 170°C to react for 2 hours. Afterwards, it was cooled to room temperature, centrifuged at 13000 rpm, and the sample of the separated solid part was washed 4 times with a mixed organic solvent of hexane and ethanol at a volume ratio of 2:1, and then dried under vacuum to obtain One-dimensional PtNiGaRe alloy nanowire materials.
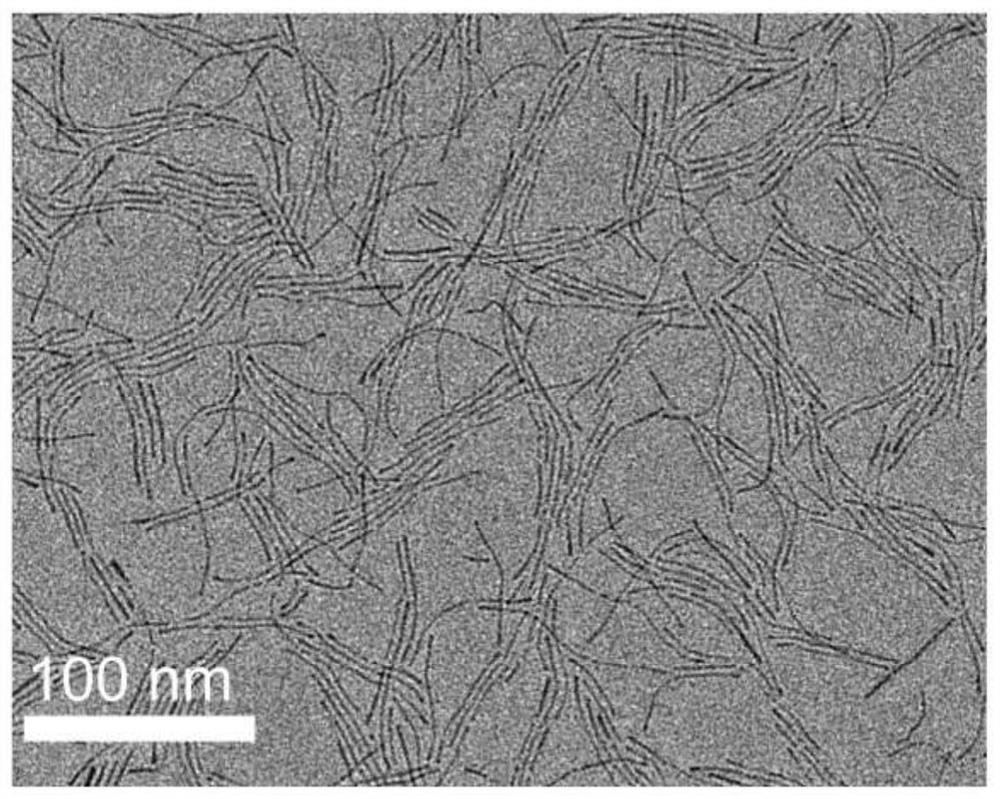
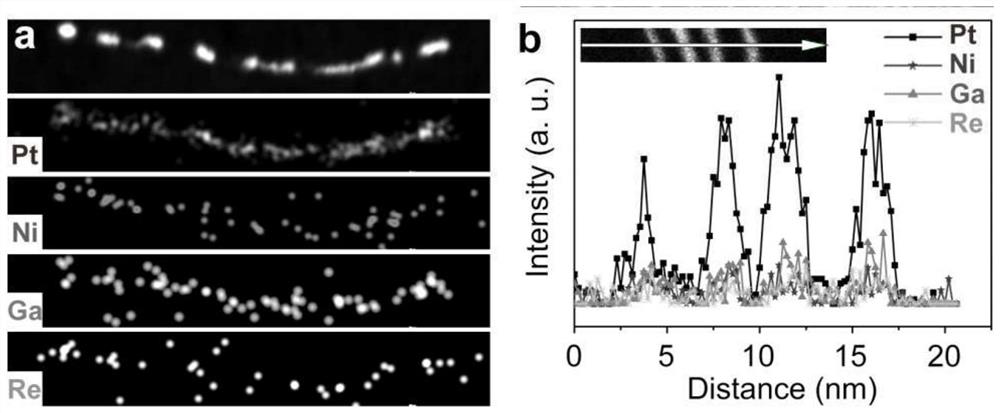
[0021] figure 1 Transmission electron microscope (TEM) image of the one-dimensional PtNiGaRe quaternary alloy nanow...
Embodiment 2
[0024] Weigh 4.0 mg of the one-dimensional PtNiGaRe alloy nanowire material prepared in Example 1, dissolve it in 8 mL of chloroform solution, and ultrasonically treat it for 1 h, add the above uniformly dispersed solution dropwise to the ethanol solution containing 16 mg of carbon black and stir vigorously 30 min; centrifuge the resulting mixture at 10,000 rpm, wash the separated solid sample twice with hexane, redisperse it in acetic acid, and heat at 70°C for 12 h to remove the surface activity on the nanowire surface Afterwards, by centrifugation, the separated solid sample was washed 5 times with ethanol, and dried to obtain a carbon-supported one-dimensional PtNiGaRe alloy nanowire material catalyst (ie, PtNiGaRe / C nanocatalyst).
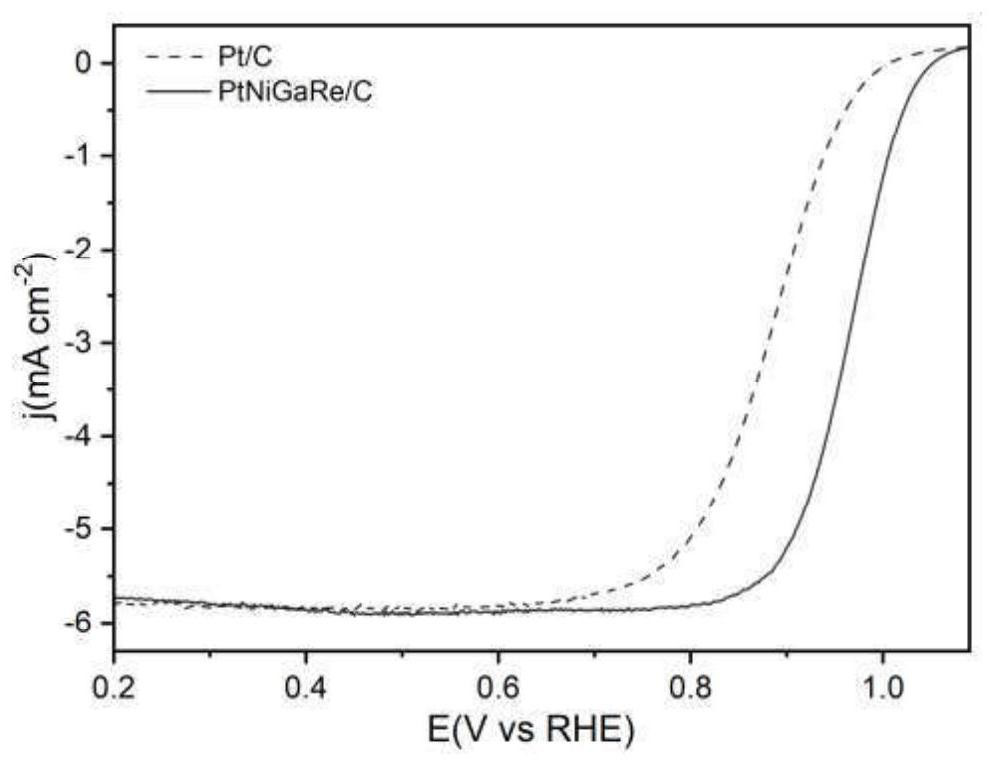
[0025] In order to compare the catalytic activity of the catalyst in this example, the PtNiGaRe / C nano-catalyst of Example 2 and the existing Pt / C catalyst were tested under the same test conditions, and the three-electric system was used to ca...
Embodiment 3
[0028] Weigh 5 mg of the PtNiGaRe / C nanocatalyst obtained in step 2 of Example 2 and mix it with 1.16 mL of isopropanol, 0.289 mL of deionized water and 0.054 mL of Nafion alcohol solution, and ultrasonicate for 1 hour to form a uniformly mixed catalyst ink solution. The above catalyst ink solution was sprayed on carbon paper, used as the cathode catalyst of the fuel cell membrane electrode assembly, and the fuel cell performance test of the one-dimensional PtNiGaRe alloy nanowire was carried out.
[0029] Continuously test the electrical performance of the above-mentioned fuel cell at a constant potential of 0.75V for 100h, the results are as follows Figure 5 shown. It can be seen from the figure that the attenuation of the output current density of the above-mentioned fuel cell using the PtNiGaRe / C nano-catalyst is almost negligible, and only drops by 4.9%, showing excellent stability.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com