Mesalazine enteric-coated tablet composition with four-layer coating system and preparation method of mesalazine enteric-coated tablet composition
A technology of mesalazine and salazine tablets, which is applied in the field of medicine and can solve problems such as large dose, inability of medicine to be absorbed by mucous membranes, poor stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
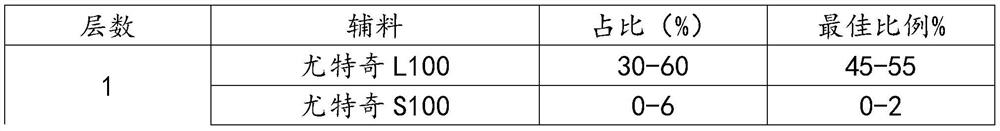
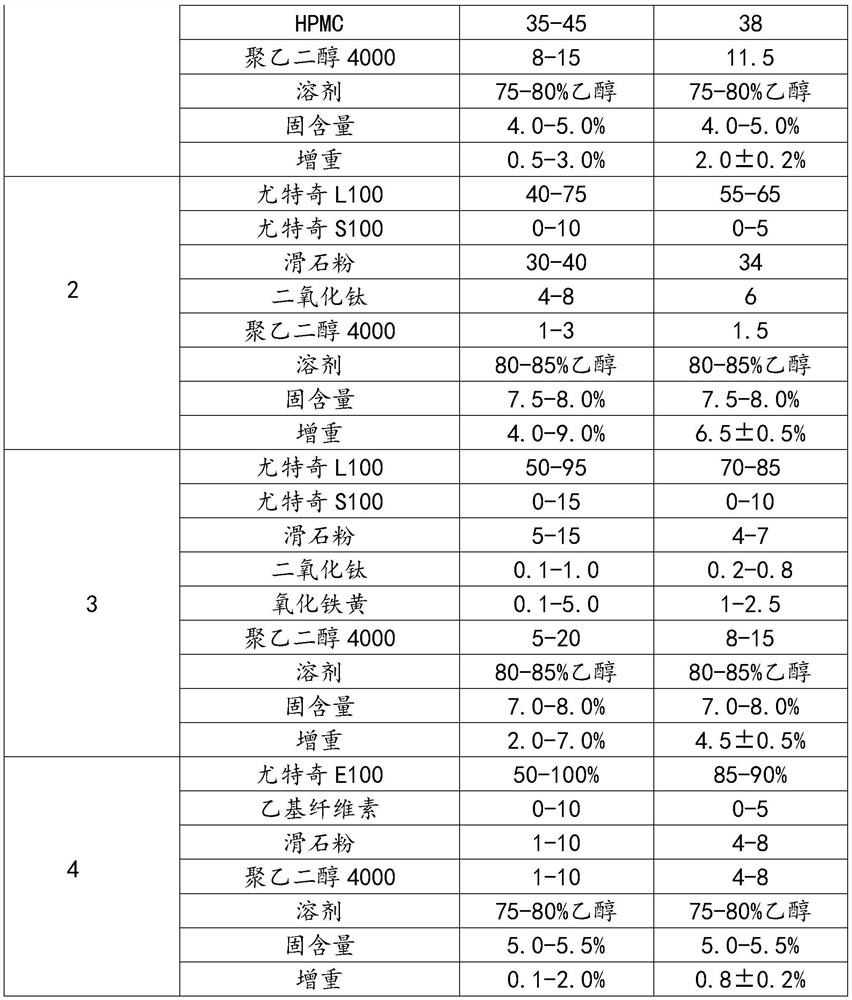
[0104] (1) prepare the mesalamine tablet core (10000 pieces) of specification 250mg, and its batching is as shown in table 3:
[0105] table 3
[0106] Name of raw material Weight (g) Mesalazine 2500 Glycine 100 Sodium carbonate 1100 microcrystalline cellulose 500 povidone 200 talcum powder 50 Calcium stearate 50
[0107]Glycine and sodium carbonate were crushed separately, glutamic acid was passed through a 80-mesh sieve, and mesalazine was crushed through a 60-mesh sieve. Accurately take by weighing 2500g of mesalamine (dry to pure, that is, feeding amount=prescription amount÷content÷(1-moisture)), glycine 100g, microcrystalline cellulose 500g, anhydrous sodium carbonate 1100g. Put it into a high-speed mixing granulator, stir at a low speed, shear at a high speed, and pre-mix for 15 minutes. Weigh Povidone K 30 200g, stir and dissolve with 480g of 95% ethanol and 720g of purified water to make 14% povidone K 30 40...
Embodiment 2
[0150] (1) The preparation specification is 500 mg mesalazine tablet core (10000 tablets): the proportioning ratio of its primary and auxiliary materials is shown in Table 10:
[0151] Table 10
[0152] Name of raw material Weight (g) Mesalamine 5000 Glycine 100 Anhydrous Sodium Carbonate 1100 microcrystalline cellulose 500 povidone 400 talcum powder 50 Calcium stearate 50 Croscarmellose Sodium 20
[0153] Glycine and anhydrous sodium carbonate were crushed separately, glutamic acid was passed through an 80-mesh sieve, and mesalazine was crushed through a 60-mesh sieve. Accurately take by weighing mesalamine 5000g (dry to pure, that is, feeding amount=prescription amount÷content÷(1-moisture)), glycine 100g, microcrystalline cellulose 500g, anhydrous sodium carbonate 1100g. Put it into a high-speed mixing granulator, stir at a low speed, shear at a high speed, and pre-mix for 20 minutes. Weigh Povidone K 30 400g...
Embodiment 3
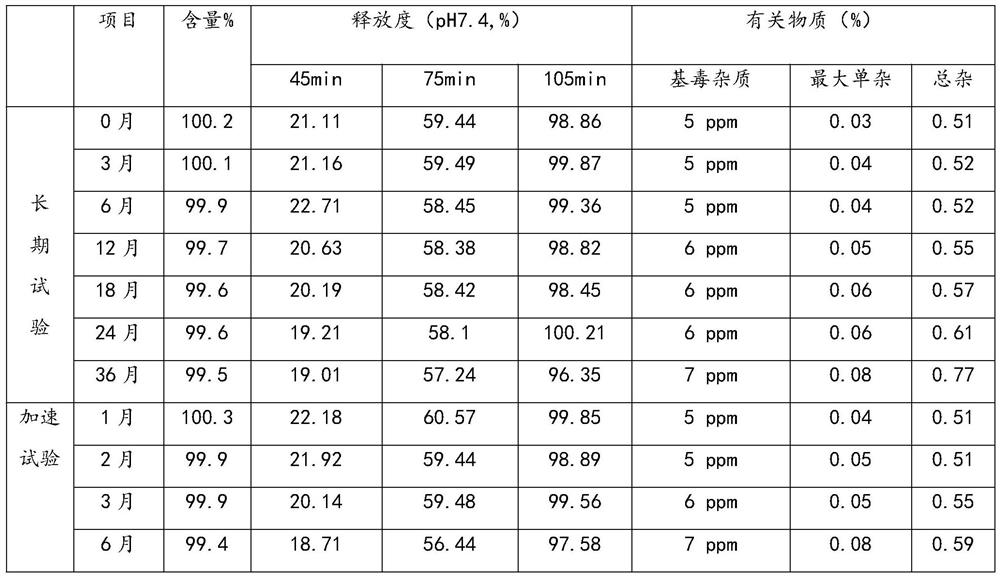
[0191] Mesalazine enteric-coated tablets with a specification of 250 mg were prepared by using the preparation method in Example 1 and according to the proportions in Table 16. The stability of the prepared samples is shown in Table 17.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com