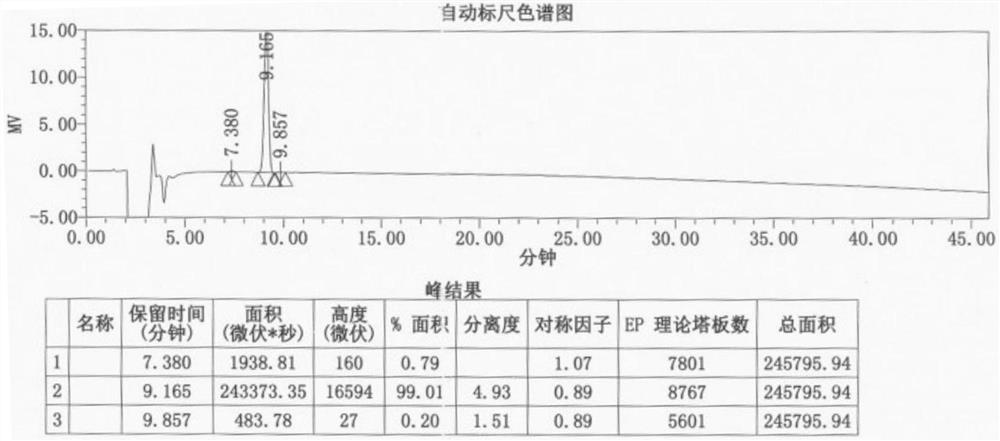
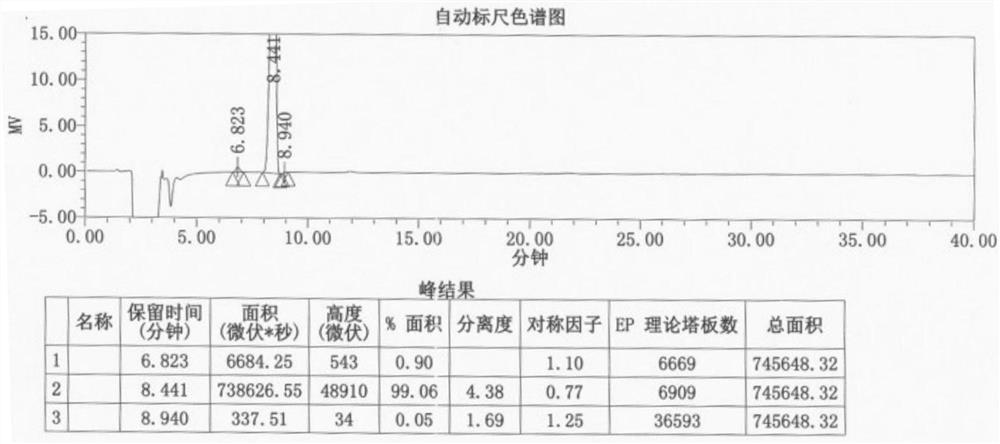
Preparation method of lithocholic acid and intermediates thereof
A technology for lithocholic acid and intermediates, which is applied in the field of synthesis of lithocholic acid and its intermediates, and can solve the problems of large material loss, difficulty, and cost increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Embodiment 1 lithocholic acid synthetic method (synthetic route one)
[0085]
[0086] Add 20g of compound I, 100ml of tetrahydrofuran, 100ml of absolute ethanol, and 0.2g of piperazine into the reaction flask, dissolve it under stirring, and then add 2g of 10% Pd / C. The system was first replaced with nitrogen, then replaced with hydrogen, and finally hydrogenated at normal pressure to complete the reaction. After the reaction was completed, the system was replaced with nitrogen for hydrogen, and palladium carbon was filtered off. The filtrate was transferred to a reaction kettle and concentrated to a viscous state. Silica gel column chromatography, gradient elution, petroleum ether: ethyl acetate = 20:1 ~ 5:1, combined the pure products, and dried to obtain 17.2g of compound II, the yield 85.5%.
[0087] Add 45g of compound II, 45g of trimethyl orthoacetate, 5g of anhydrous methanol, and 2.2g of p-toluenesulfonic acid into the reaction flask, and stir the reaction...
Embodiment 2
[0093] Embodiment 2 synthetic method of lithocholic acid (synthetic route two)
[0094]
[0095] Add 100g of BA dissolved in 500ml of dichloromethane into the reaction bottle, add 1g of TEMPO, 5g of sodium bicarbonate, 4g of sodium bromide, cool down to -5 ~ 5 degrees, add dropwise 250ml of 9-10% sodium hypochlorite, dropwise, at 0 ~ React at 10 degrees for 1 to 2 hours. Take samples for TLC until the reaction is complete. Control the temperature at 0-10 degrees and add an aqueous solution of sodium thiosulfate dropwise until it is non-oxidizing, stir, separate layers, extract the water layer with dichloromethane, wash the combined organic layer with sodium thiosulfate, water, and saturated brine , concentrated under reduced pressure until a large amount of yellow solid precipitated, added acetonitrile twice, continued to concentrate under reduced pressure, added acetonitrile, stirred at room temperature, filtered, and dried the filter cake to obtain 96 g of compound F, wi...
Embodiment 3
[0099] Embodiment 3 lithocholic acid synthetic method (synthetic route three)
[0100]
[0101] Add 100g of BA dissolved in 500ml of dichloromethane into the reaction bottle, add 1g of TEMPO, 5g of sodium bicarbonate, 4g of sodium bromide, cool down to -5 ~ 5 degrees, add dropwise 250ml of 9-10% sodium hypochlorite, dropwise, at 0 ~ React at 10 degrees for 1 to 2 hours. Take samples for TLC until the reaction is complete. Control the temperature at 0-10 degrees and add an aqueous solution of sodium thiosulfate dropwise until it is non-oxidizing, stir, separate layers, extract the water layer with dichloromethane, wash the combined organic layer with sodium thiosulfate, water, and saturated brine , concentrated under reduced pressure until a large number of yellow solids precipitated, added acetonitrile twice, continued to concentrate under reduced pressure, added acetonitrile, stirred at room temperature, filtered, and dried the filter cake to obtain 96 g of compound F, wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com