Biliverdin compound as well as preparation method and application thereof
A compound and biliverdin technology, applied in the field of drug synthesis, can solve the problems of difficult separation, many by-products and high cost, and achieve the effects of simple preparation process, low cost and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056]Method for preparation of chlorinated compounds
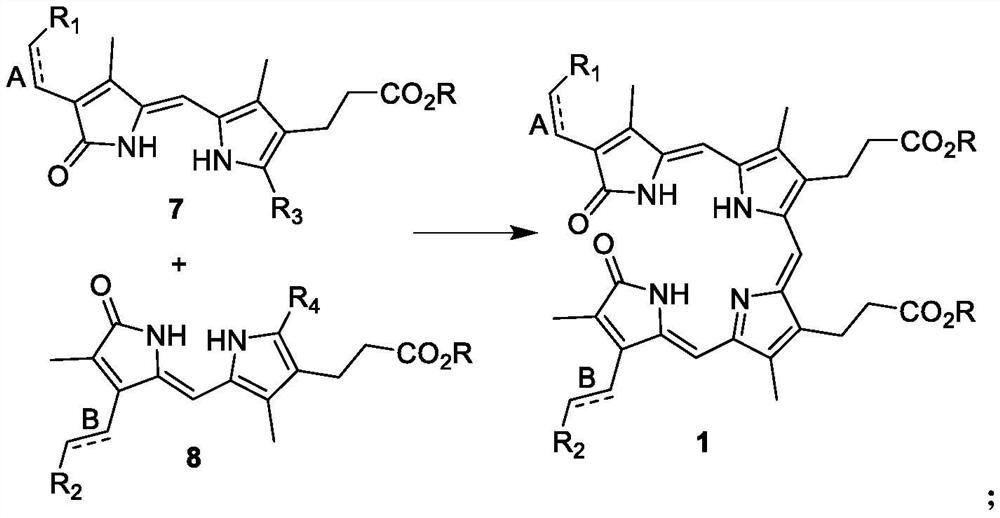
[0057]The present invention also provides a method of preparation of the above-mentioned chlorophyll intermediate, which is obtained by condensation by the compound of formula 2 and the compound of formula 3, and the reaction formula is as follows:
[0058]
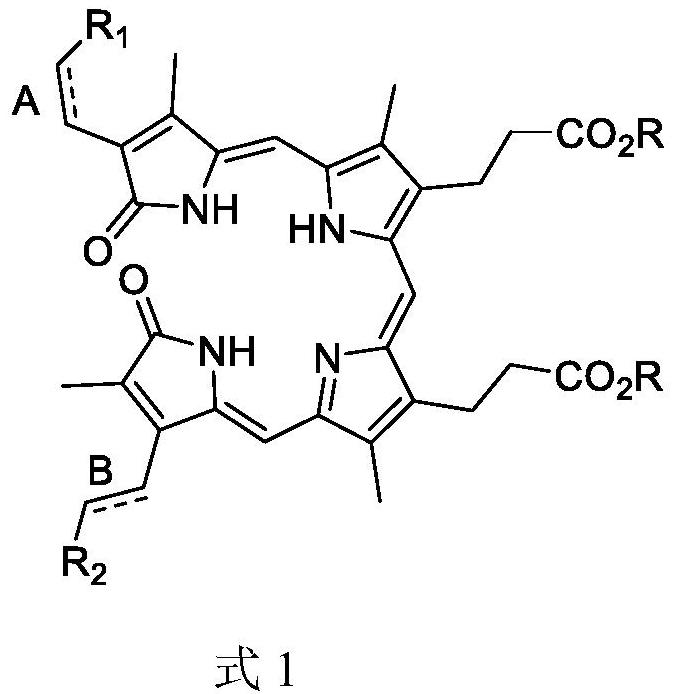
[0059]Among them, R is selected from hydrogen, c1~ C5One of alkyl and benzyl groups;Indicates the two-button or single key, the position shown in A, BIndependently selected from one of the single bonds and double bonds,When it is a single bond, R is connected to the single button.1Or R2The toluene group is selected from the group consisting of toluene groups, toluene sulfonyl, phenyl sulfone group, benzulfonyl;When the double button is connected to the double button1Or R2One of hydrogen, R3 and R4 is aldehyde group, R3R4Another one selected from the group consisting of tert-butoxycarbonyl, hydrogen.
[0060]In the technical solution of the invention, the condensation reaction is carri...
Embodiment 1
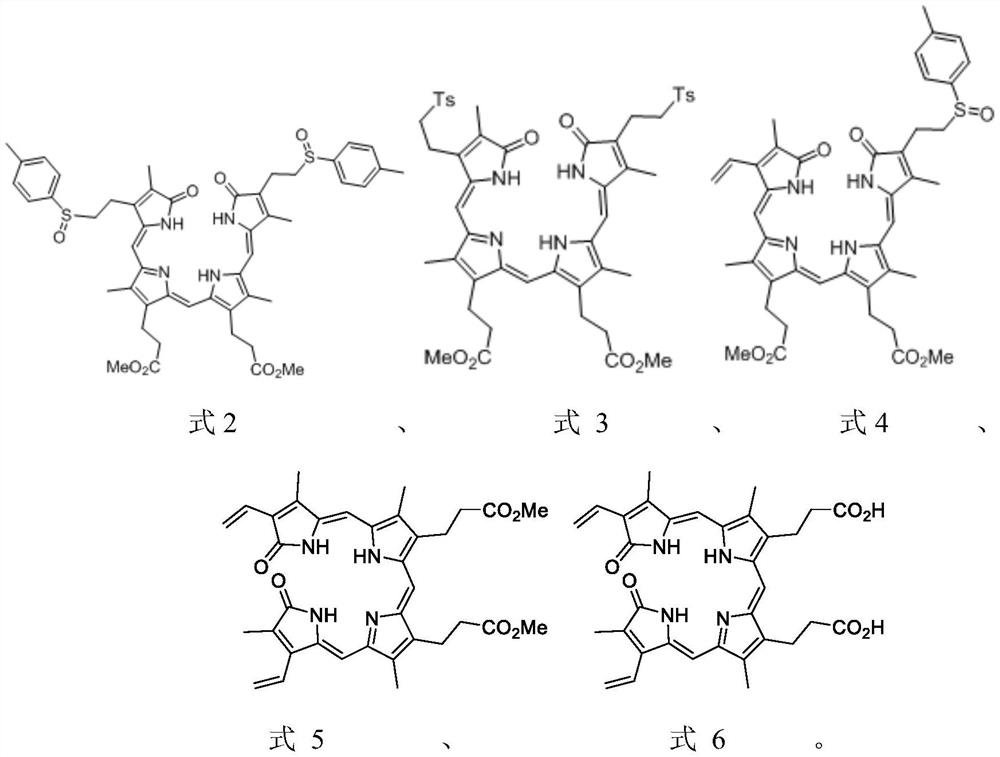
[0088]Examples 1: 3, 3 '- (3,18 - 2 (2 - Toluealesulfonylthyl) -2, 7, 13, 17-tetramethyl-1, 19-dioxidation-1,19 , 22, 24-tetrahydro-21H-8, 12-porphyrin group) - Synthesis of dimethyl propionate (compound of formula 2)
[0089]
[0090]Weigh 1.00 gram of 9-tert-butoxycarbonyl-3,7-dimethyl-8- (2-methoxycarbonylthyl) -2- (2-p-tolueraulfonylthyl)-dipyrid Ethylene-1-keto (compound of formula 9), dissolved with 5 ml of trifluoroacetic acid, stirred at a temperature of 25 ° C for 30 minutes, add 0.87 grams of compound 9-formyl-2,7-dimethyl-8 - (2-methoxycarbonyl) -3- (2-piocarbylsulfonylthyl) - dipyrrolidate-1-ketone (compound of formula 10), stirred at a temperature of 25 ° C for 10 hours , 20 ml of dichloromethane was added, washed out of the organic layer, saturated sodium hydrogencarbonate to neutral, dried over anhydrous sodium sulfate, filtration, ethanol refined crystal blue green solid 0.85 grams, 3, 3, 3, 3, 18 - Second (2-p-toluenesulfonylthyl) -2, 7, 13, 17-tetramethyl-1,19-dioxo-1, 1...
Embodiment 2
[0091]Example 2: 3, 3 '- (3,18-2 (2-p-tolueraulfonylthyl) -2, 7, 13, 17-tetramethyl-1, 19-dioxide-1,19 , 22, 24-tetrahydro-21H-8, 12-porphyrin group) - Synthesis of dimethyl propionate (compound of formula 2)
[0092]
[0093]1.00 grams of compound-3,7-dimethyl-8- (2-methoxycarbonylthyl) -2- (2-p-toluenesulfonylthyl)-dipyridolin-1-ketone ( 11 compound compounds) and 0.87 g of 9-formyl-2,7-dimethyl-8- (2-methoxycarbonylthyl) -3- (2-p-toluearsulfonylthyl)-dipyrid Ethylene-1-ketone (shown in Formula 10), after mixing, dissolved with 50 ml of methanol, then add 1.5 ml of 1M hydrochloride solution, stirring at a temperature of 15 ° C for 10 hours, concentrated under reduced pressure, dichloromethane dissolved, Wash the saturated sodium bicarbonate to neutral, dry dry, filtrate, filtration, and ethanol, crystal crystal, 5,3 '- (2 - 2 - 2 - 2 - 2-p-Toluene) Sulfonylthyl) -2, 7, 13, 17-tetramethyl-1, 19-dioxo-1, 19, 22, 24-tetrahydro-21H-8, 12-porphyrin group) -bu Dimethyl propionate (compound of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com