Method for simultaneously determining eight antituberculous drugs in human plasma based on LC-MS
An LC-MS and anti-tuberculosis technology, applied in the field of analytical chemistry, can solve the problems that chromatography is difficult to meet the requirements of low-concentration drug determination, biological matrix interference drug determination, and low drug concentration, so as to save analysis costs, ensure accuracy, and improve health. Minimal health hazard effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Chromatographic conditions used:
[0042] Chromatographic column: the chromatographic column used is ACQUITY UPLC HSS T3 1.8μm column (2.1×100mm, Waters) ultra-high performance liquid chromatography column;
[0043] Column temperature: 35°C
[0044] Sample tray temperature: 4°C
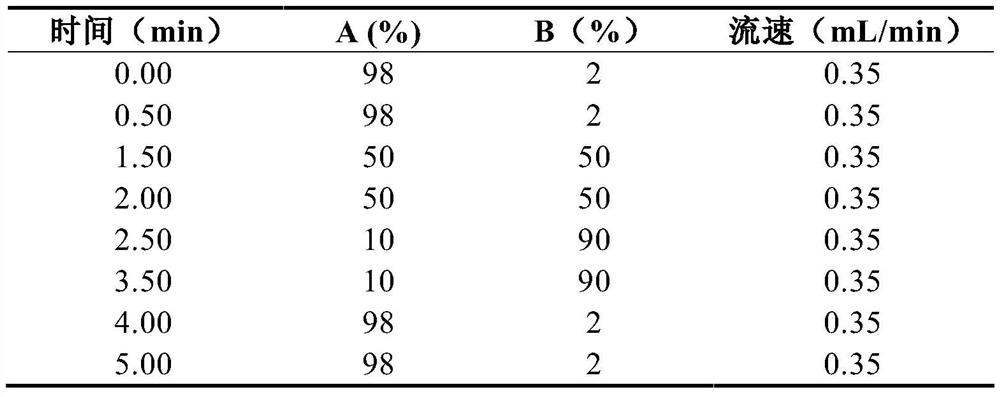
[0045] Flow rate: 0.35mL / min
[0046] Injection volume: 5μL
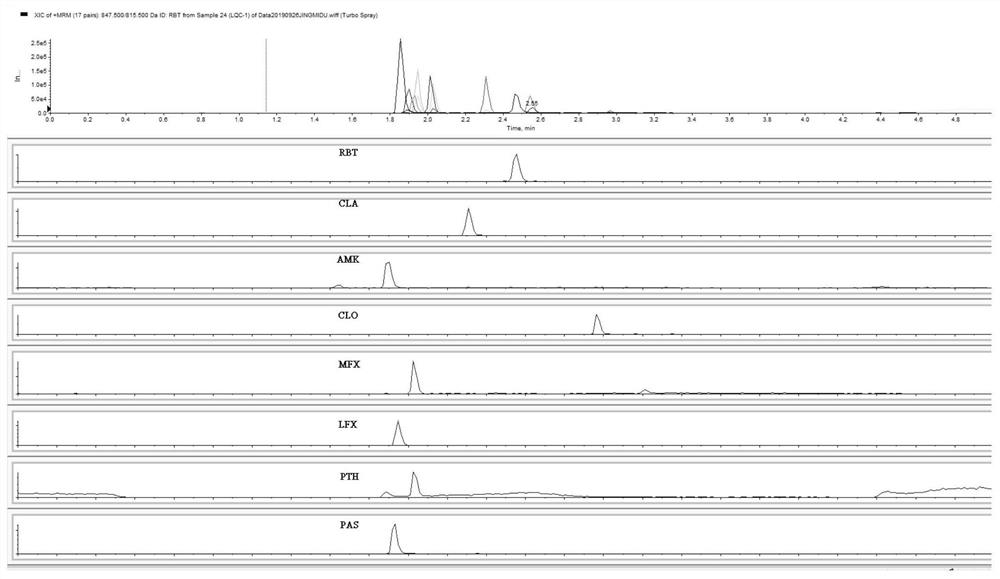
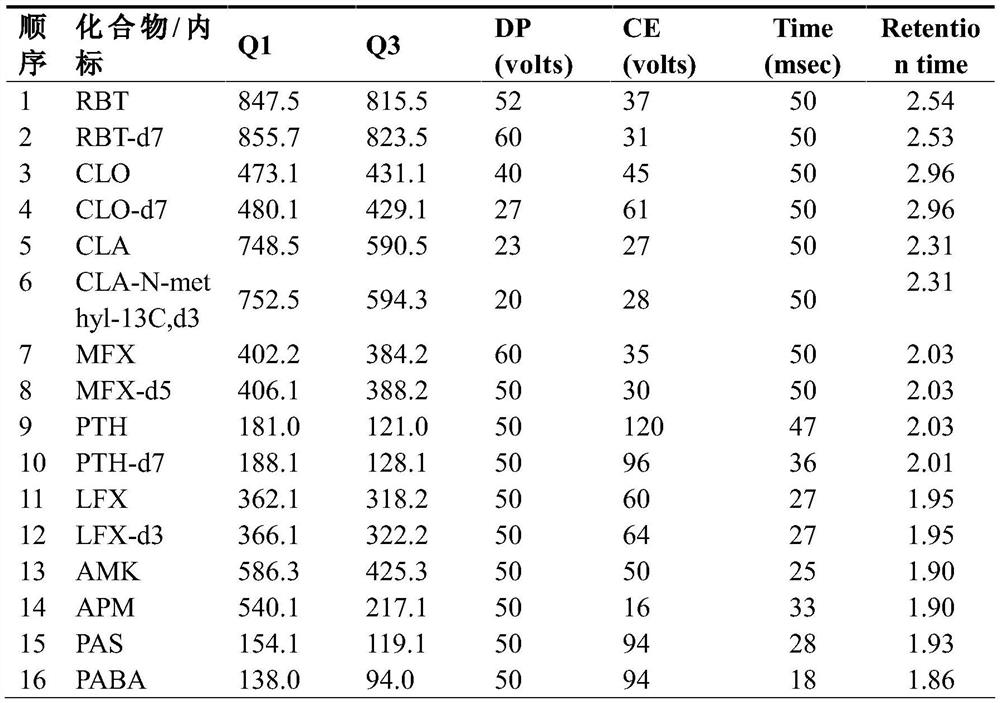
[0047] The retention time of each compound is shown in Table 2.
[0048] Mass Spectrometry Conditions:
[0049] Scanning method: multiple reaction ion monitoring (MRM)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com