Preparation method and application of fluorescent probe based on benzothiazole Schiff base
A technology of benzothiazoles and fluorescent probes, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of high cost, harsh reaction conditions, and poor sensitivity of zinc ion molecular fluorescent probes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Synthesis of compound a: 2-aminothiophenol (2g, 16.1mmol) and 2-hydroxy-5-methylbenzaldehyde (2.45g, 16.1mmol) were added to a round-bottomed flask containing 20mL of anhydrous methanol, Add I to the solution 2 (2.04g, 8.05mmol), the mixture was heated to reflux for 4h under the protection of nitrogen. After the reaction was completed, it was cooled to room temperature, and a yellow solid precipitated out. The solid was collected, filtered with a Buchner funnel and washed with cold methanol. It was further dried in vacuo to obtain a pale yellow solid, namely compound a (1.63 g, 42.1%). The characterization results are as follows: 1 H NMR (600MHz, CDCl 3 )δ12.21(s,1H),7.92(d,J=8.2Hz,1H),7.84(d,J=8.0Hz,1H),7.54–7.43(m,2H),7.34(t,J=7.2 Hz, 1H), 7.19 (d, J=8.4Hz, 1H), 7.13 (d, J=8.4Hz, 1H), 2.29 (s, 3H).
[0035] Synthesis of compound b: Compound a (1.40g, 5.82mmol), hexamethylenetetramine (1.22g, 8.73mmol) were added to a round-bottomed flask containing 8mL trifluoroa...
Embodiment 2
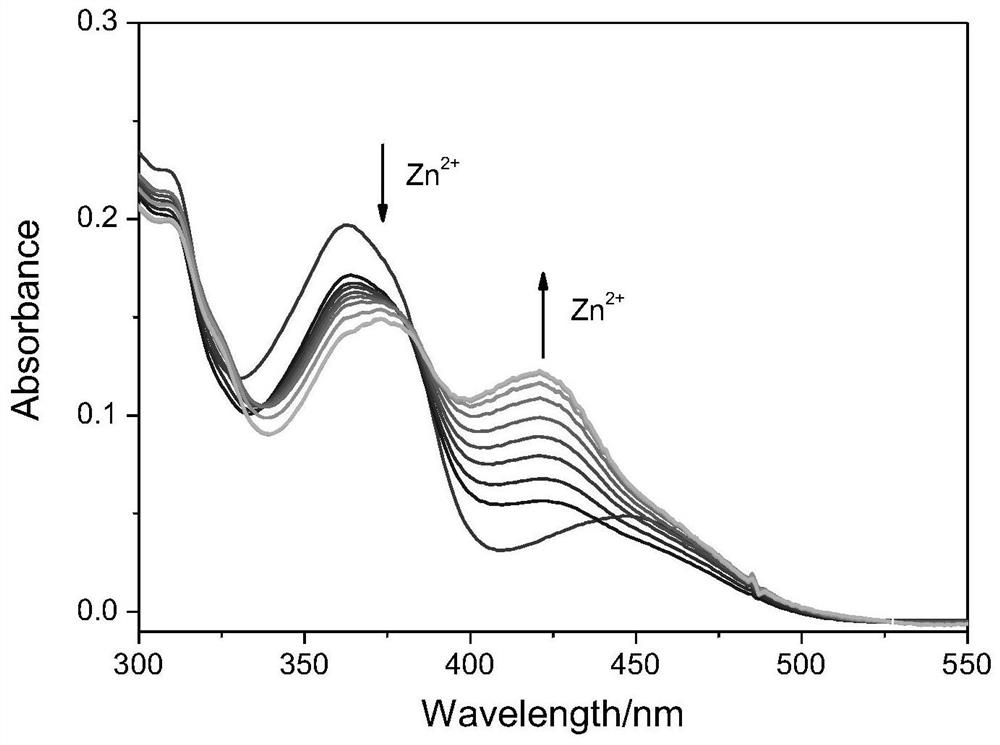
[0038] Probe molecule with Zn 2+ UV absorption spectrum of concentration change
[0039] To test the probe molecules for different concentrations of Zn 2+ Under the same experimental conditions, a buffer solution of 1mL absolute ethanol and Tris-HCl (CH 3 CH 2 OH:Tris-HCl=3:2, V / V, 20mmol, pH=7.4) was added to 10μL probe molecule stock solution (1mmol / L) for Zn 2+ UV titration experiment, and test its UV absorption spectrum ( figure 1 ). Depend on figure 1 It can be seen that adding Zn 2+ Afterwards, a new absorption peak appeared at 421nm for the probe molecule, and as the concentration of zinc ions in the solution gradually increased, the absorption peak at 421nm gradually increased, and at the same time, the absorption peak at 363nm for the probe molecule red-shifted to 373nm. An isoabsorptive point appears at 383nm.
Embodiment 3
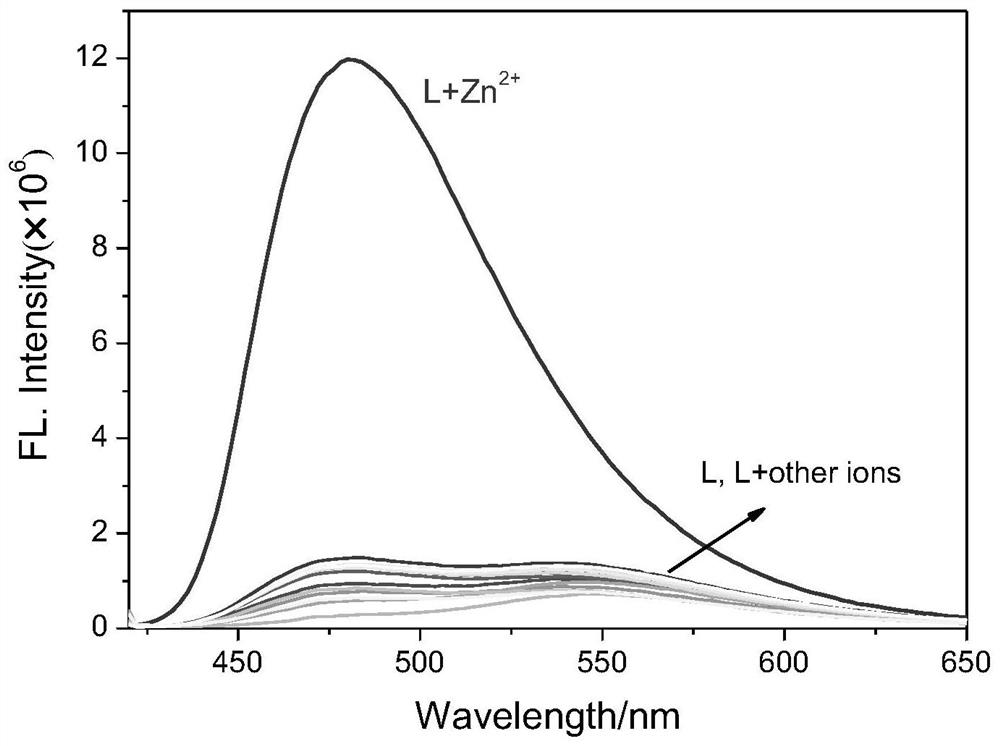
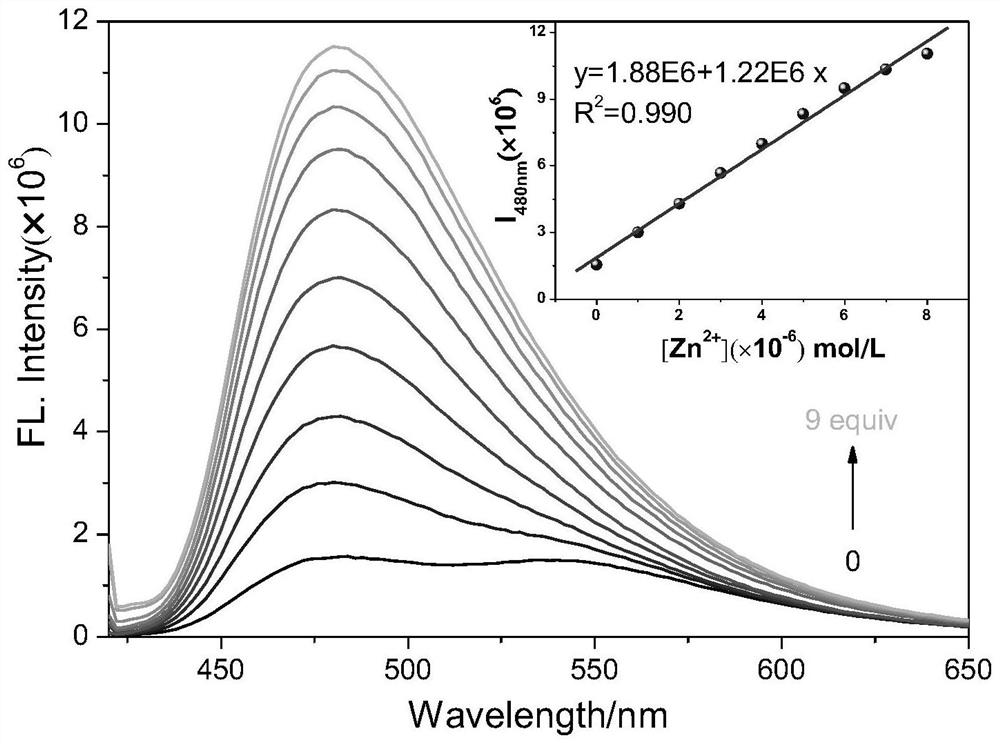
[0041] Probe molecules and Zn 2+ Fluorescence spectrum research before and after the action
[0042] For testing probe molecules with Zn 2+ Fluorescence spectra before and after the action, under the same experimental conditions, to 1mL absolute ethanol and Tris-HCl buffer solution (CH 3 CH 2 OH:Tris-HCl=3:2, V / V, 20mmol, pH=7.4), add 1μL probe molecule stock solution (1mmol / L), then drop 1μL different metal ion stock solution (10mmol / L), test Its fluorescence spectrum ( figure 2 ). Depend on figure 2 It can be seen that only adding Zn to the probe molecule solution 2+ After that, the emission peak at 480nm was significantly enhanced, accompanied by an obvious fluorescence color change from yellow to blue, indicating that the probe molecule could selectively detect zinc ions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com