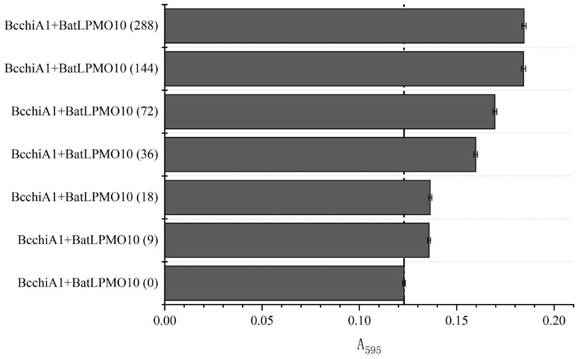
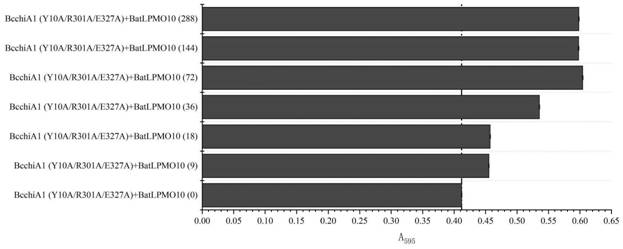
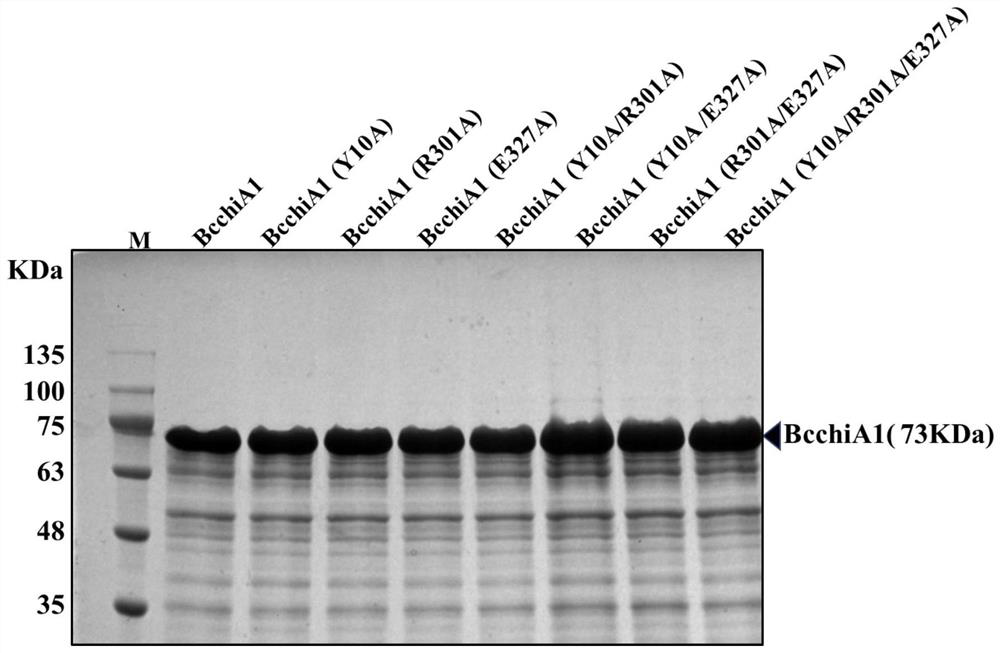
Construction of recombinant bacterium capable of efficiently expressing chitinase and screening of mutant with high enzyme activity
A chitinase and mutant technology, applied in the field of genetic engineering, can solve the problems of insufficient secretion and low activity of wild-type chitinase, achieve considerable industrial application value, improve flux and efficiency, and enhance hydrolysis efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Construction of pMATE-BcchiA1 recombinant plasmid and recombinant bacteria
[0027] 1. Construction of pMATE-BcchiA1 recombinant plasmid
[0028]Chitinase BcchiA1 from Bacillus circulans WL-12 has strong affinity and catalytic activity for highly insoluble chitin. Entrust Suzhou Jinweizhi Biotechnology Co., Ltd. to artificially synthesize the gene after optimization according to the codon preference, and add a His tag to the C-terminus for protein purification. Its nucleic acid sequence is shown in SEQ ID NO.1, and its amino acid sequence is shown in SEQ ID NO. 2, the gene was synthesized and cloned into the vector pUC57-Amp to obtain the recombinant plasmid pUC57-BcchiA1. The recombinant plasmid pUC57-BcchiA1 was used as a template, and a primer pair composed of primer FragmentF and primer FragmentR was used for PCR amplification to obtain a BcchiA1 fragment of about 1.9 kb. Using the pMATE plasmid as a vector template, the vector backbone was amplified fro...
Embodiment 2
[0034] Embodiment 2 develops the new method of directed evolution of chitinase
[0035] 1. Construction of chitinase mutation library
[0036] Using the wild-type BcchiA1 nucleic acid sequence shown in SEQ ID NO.1 as a template, through the TIANZ adjustable error-prone PCR kit, use primers Fragment-F and Fragment-R to amplify the target gene, and introduce random mutations into the BcchiA1 gene (1.9kb). The error-prone PCR reaction system is shown in Table 2. The error-prone PCR reaction program is: 94°C, 3min; 94°C for 1min, 45°C for 1min, 72°C for 2min, 50 cycles.
[0037] Table 2
[0038] Element Volume (μL) Error-prone PCRMix, 10x 3.0 dNTP for error-prone PCR, 10 3.0 MnCl for error-prone PCR 2
1.0 dGTP for error-prone PCR 0 BcchiA1 template (5ng / μL) 3.0 Fragment-F / R(10Meach) 1.0 TaqDNA polymerase for error-prone PCR 0.5 Ultra-pure water 17.5
[0039] The PCR product that has introduced random mutations...
Embodiment 3
[0042] Example 3 High enzymatic activity single mutation site further superposition-site-directed mutation
[0043] 1. Single-point mutants Obtain two-point superposition mutants through site-directed mutation
[0044] 1. The acquisition of mutant BcchiA1 (Y10A / R301A): with the single-point mutant BcchiA1 (Y10A) as a template, the primer pair consisting of primers R301A-F and primer R301A-R shown in Table 3 was used for PCR amplification to obtain BcchiA1 (Y10A / R301A) fragment of about 1.9 kb. After purification by the Gel Recovery Kit, pass the kit The completed fragment was seamlessly connected with the vector backbone pMATE in Example 1, and transformed into Escherichia coli DH5α competent cells. After sequencing, the construction of the recombinant plasmid pMATE-BcchiA1(Y10A / R301A) was completed.
[0045] 2. The acquisition of mutant BcchiA1 (Y10A / E327A): with the single-point mutant BcchiA1 (Y10A) as a template, the primer pair consisting of primers E327A-F and primers...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com