Cell model for screening in-vitro activity of URAT1 inhibitor as well as construction method and screening method of cell model
A cell model and construction method technology, applied in the field of pharmacology and genetic engineering, can solve the problems of harmful environment, pollution, high cost of use, etc., and achieve the effect of high specificity, high sensitivity and low requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
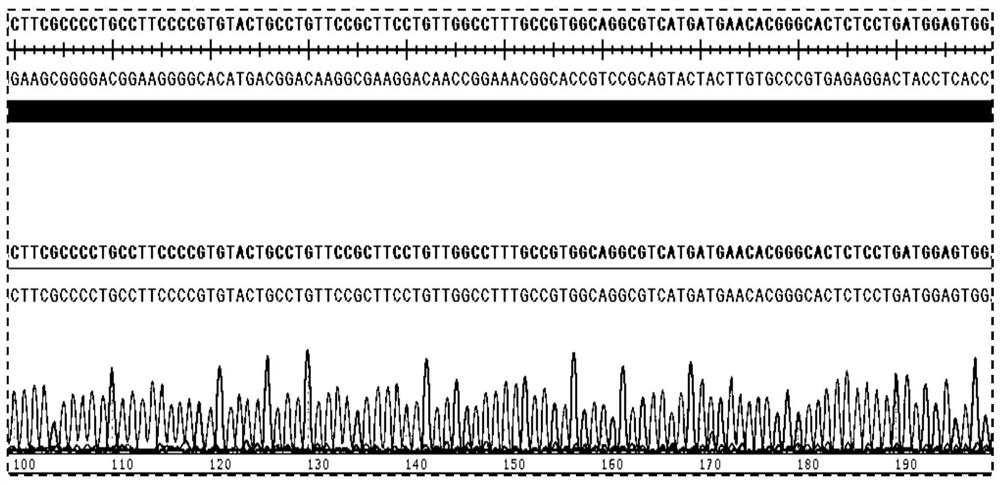
[0065] Example 1 Constructing a cell line that stably and highly expresses the hURAT1 gene
[0066] 1. Preparation of related solutions and reagents:
[0067] (1) Ampicillin liquid storage solution: Accurately weigh 0.5 g of ampicillin powder with an analytical balance, place it in a 15 mL centrifuge tube, add 3 mL of distilled water to dissolve it, and then dilute to 5 mL to obtain ampicillin with a final concentration of 100 mg / mL penicillin stock solution. Filter and sterilize with a 0.22 μm nitrocellulose membrane in an ultra-clean bench, aliquot and store in a -20°C refrigerator. When in use, dilute the ampicillin stock solution to a working concentration of 100 μg / mL according to the volume of the medium.
[0068] (2) Complete medium for HEK293T cells: Add 10wt% fetal bovine serum, 1wt% penicillin / streptomycin and 1wt% L-glutamine to DMEM high-glucose basal medium, mix well and filter with 0.22 μm nitrocellulose Sterilize by membrane filtration and store in a 4°C refr...
Embodiment 2
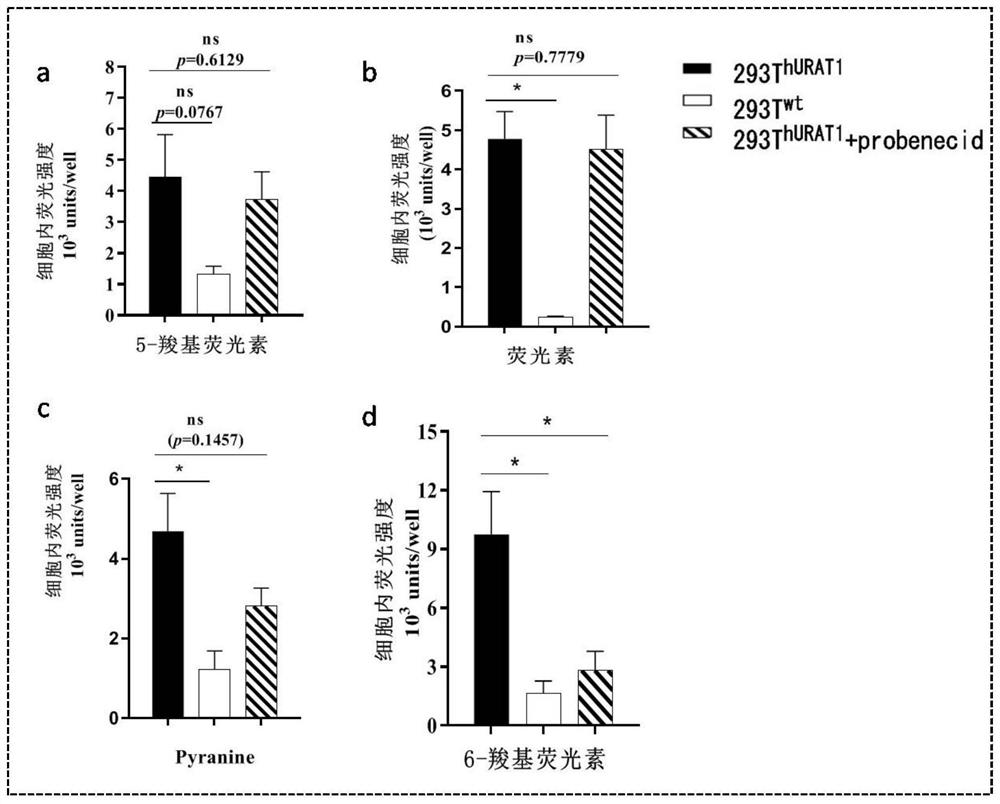
[0118] Example 2 Establishment of the in vitro activity screening model of URAT1 inhibitors based on fluorescence method
[0119] 1. Solution preparation
[0120] (1) HBSS (Cl-free - ) solution preparation: take by weighing solid sodium gluconate (125mmol / L), potassium gluconate (4.8mmol / L), calcium gluconate (1.3mmol / L), magnesium sulfate (1.2mmol / L), dihydrogen phosphate Dissolve potassium (1.2mmol / L) in a beaker with distilled water, add HEPES (25mmol / L), D-glucose (5.6mmol / L) to 1000mL, then add NaHCO 3 The pH of the solution was brought to 7.3-7.4, sterilized by filtration through a 0.22 μm nitrocellulose filter, and placed in a 4°C refrigerator for later use.
[0121] (2) Preparation of 0.5% MTT solution: Weigh 0.5g of MTT in a 100mL volumetric flask, and use prepared HBSS (Cl-free - ) solution was dissolved and fixed to volume, filtered and sterilized through a 0.22 μm nitrocellulose filter membrane, aliquoted into 15 mL centrifuge tubes and wrapped in tinfoil, seale...
Embodiment 4
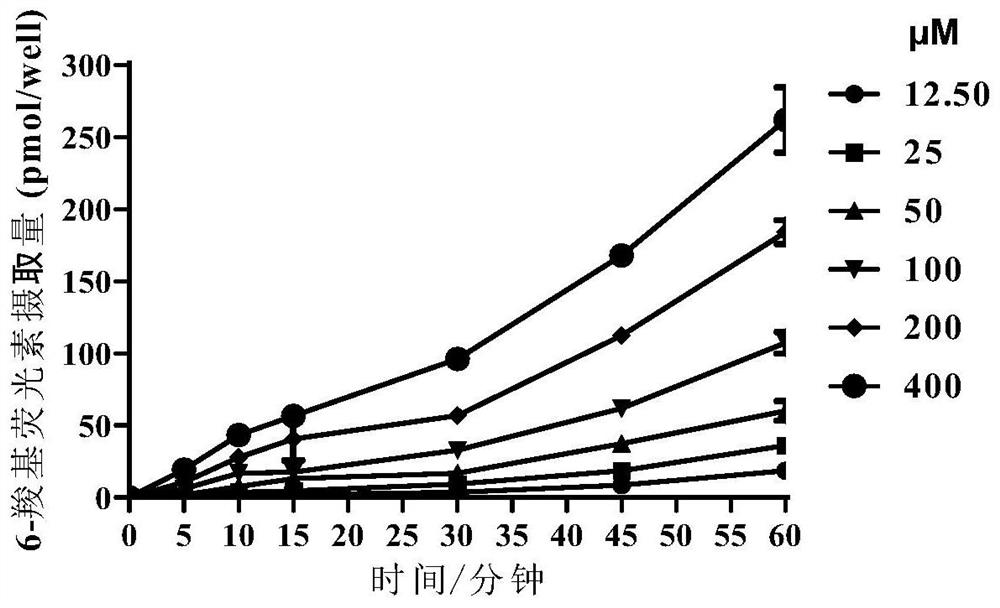
[0165] Embodiment 4 is based on the application of the in vitro screening model of fluorescence method (can refer to Figure 6 flow chart)
[0166] 1. Solution preparation
[0167] (1) Preparation of common solutions: For the preparation of HBSS (Cl-free) solution, 0.5% MTT solution, HEK293T complete medium containing 2% fetal bovine serum, fluorescent substance solution and URAT1 inhibitor solution, refer to Example 2.
[0168] (2) Preparation of test compound storage solution: Take 10 compounds, accurately weigh each test compound in a 1.5mL centrifuge tube, add 1ml DMSO to dissolve, and prepare a 200mM storage solution.
[0169] There are 10 compounds to be tested, which are marked as Compound 1, Compound 2, Compound 3, Compound 4, Compound 5, Compound 6, Compound 7, Compound 8, Compound 9, and Compound 10. Wherein, the structural formula of compound 1 is: The structural formula of compound 2 is: The structural formula of compound 3 is: The structural formula of com...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap