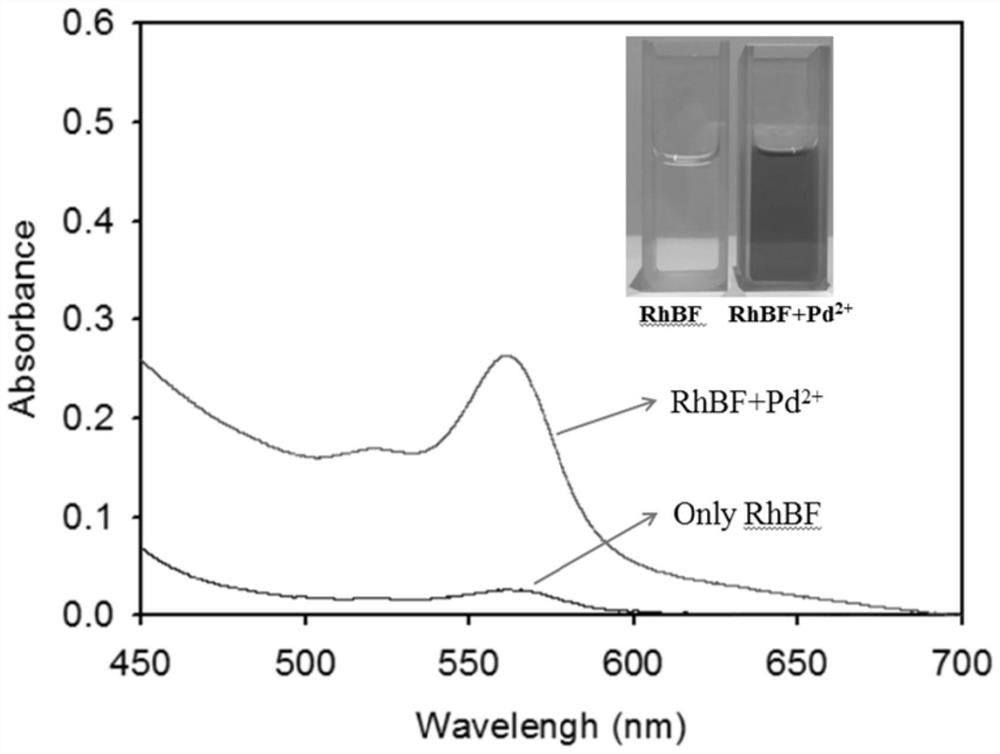
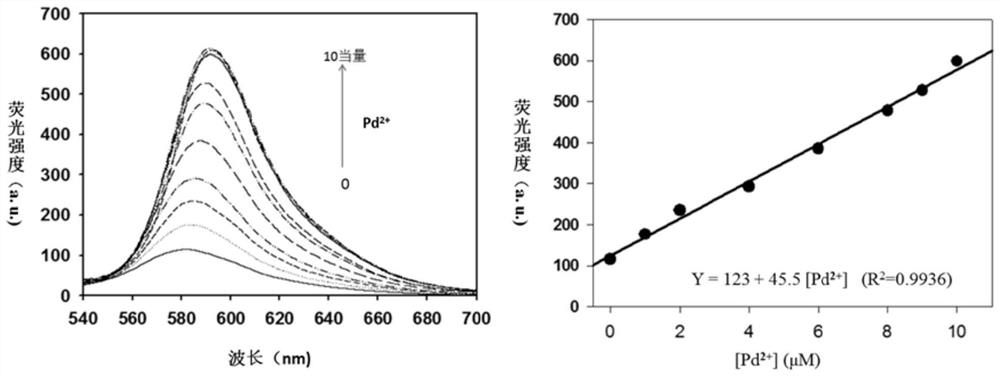
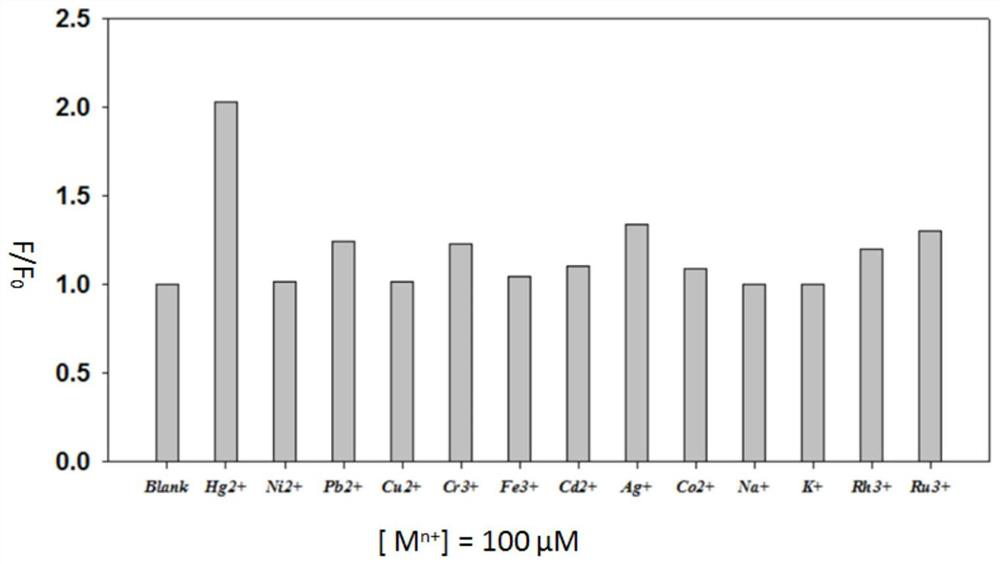
Near-infrared fluorescent probe compound taking N-pyridine oxide derivative as recognition group as well as preparation and application of near-infrared fluorescent probe compound
A pyridine oxide and fluorescent probe technology, which is applied in the field of preparation and application of new recognition groups, can solve problems such as uncertainty and unexpectedness, and achieve the effects of easy preparation, easy availability of raw materials, and simple synthesis steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Dissolve pyridinemethanol N-oxide (3.0g, 24.0mmol) in 10mL of pyridine solution, add selenium dioxide (3.20g, 28.8mmol) in batches while stirring, then heat to 90°C and reflux for 3h to stop the reaction. Suction filtration, the filter cake was washed with a small amount of dichloromethane, and the filtrate was concentrated to obtain the crude product of pyridine formaldehyde N-oxide, which was recrystallized from a mixed solvent of petroleum ether and ethyl acetate (60mL, V / V=5:1) to obtain the pure product , (2.30 g, 77.8% yield).
Embodiment 2
[0036] In a 100mL single-necked round bottom flask, add rhodamine B (2.9g, 6.0mmol), absolute ethanol (50mL) and 85% hydrazine hydrate (10mL) successively, and mix and reflux at 78°C for 12 hours, and the solution changes from dark purple to Bright yellow, and finally turned into a transparent and clear solution. After the reaction was completed, it was cooled, and the solvent was removed under reduced pressure to obtain a viscous solid. Add 10mL concentrated hydrochloric acid (1mol / L) dropwise to the above solid to obtain a red clear solution, then gradually add 1mol / L sodium hydroxide aqueous solution dropwise, adjust the pH value of the solution to 9, a large amount of precipitation occurs, filter, and deionized water The filter cake was washed and vacuum-dried to obtain rhodamine B hydrazide dyes (2.10 g, yield 76.6%).
Embodiment 3
[0038]Rhodamine B hydrazide dye (0.92g, 2.0mmol) was added to 20mL of ethanol, after it was completely dissolved, N-oxypyridineformaldehyde (0.246g, 2.0mmol) was added, and the mixture was refluxed at 80°C for 12h under the protection of nitrogen. , cooled, removed the solvent under reduced pressure, then extracted with ethyl acetate, distilled off the ethyl acetate at 45-55°C, separated by silica gel column chromatography, and the eluent was dichloromethane / methanol (V / V=30:1), to obtain The yellow solid (0.629 g, yield 56%) is the N-oxide pyridine-rhodamine B-type near-infrared fluorescent probe (RhBF). like Figure 5 , Image 6 and Figure 7 Shown, ESI mass spectrum: m / z[M+H] + 562.2799. 1 H NMR (500MHz, CD 3 Cl) δ (ppm): 1.15 (t, 12H, J = 7.0Hz), 3.32 (q, 8H, J = 7.0Hz), 6.23-6.25 (m, 2H), 6.46-6.49 (m, 4H), 7.06 -7.13(m,3H),7.46-7.54(m,2H),7.86(dd,1H,J=7.0Hz,J=2.0Hz),7.99-8.02(m,2H),9.12(s,1H); 13 C NMR (125MHz, CD 3 Cl)δ (ppm): 12.6, 44.3, 66.3, 98.1, 105.3, 107....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com