Application of nitrogen heterocyclic carbene selenium-gold compound in the preparation of drugs against carbapenem-resistant Acinetobacter baumannii
A technology of Acinetobacter baumannii and azacyclic carbene, which is applied in the field of drug preparation, can solve the problems of destruction, toxic and side effects, easy to be metabolized, etc., and achieves the effects of inhibiting infectious lesions, no toxic and side effects, and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
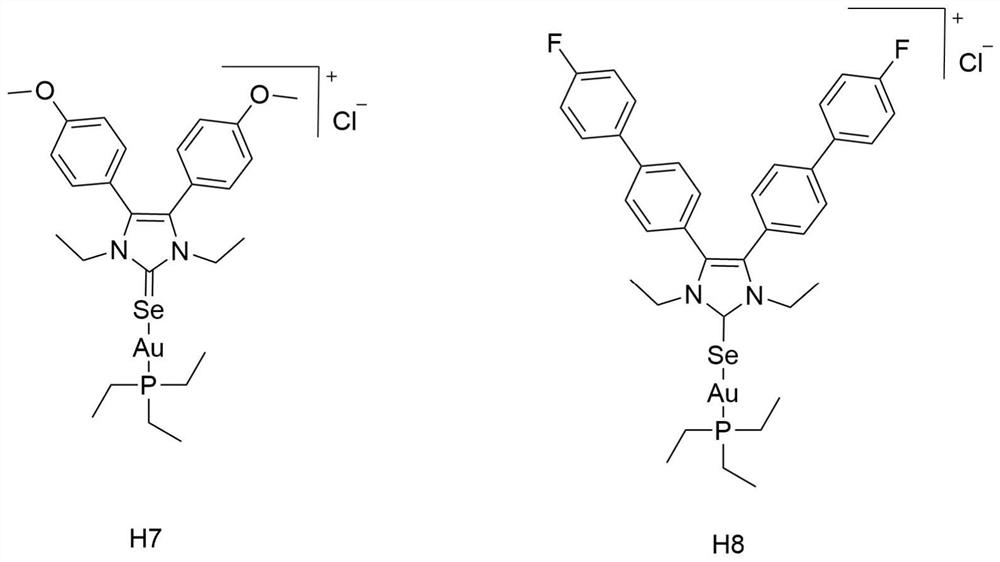
[0025] Synthesis and structural formula thereof of embodiment 1 azacyclic carbene selenium-gold compound H7 and H8
[0026] 1. Structural formula:
[0027]
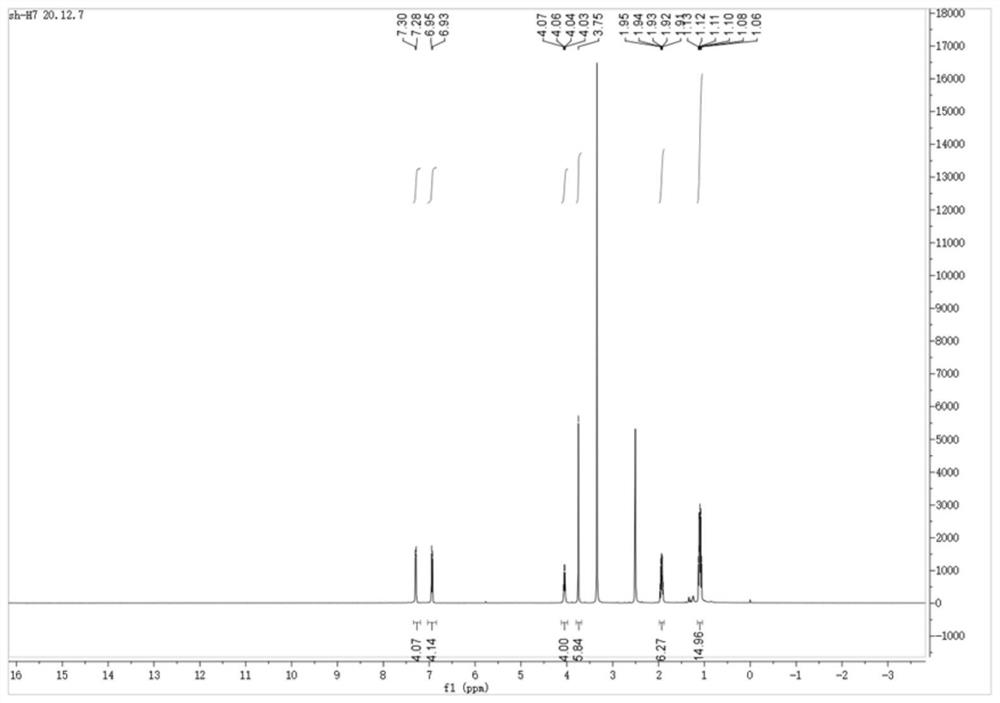
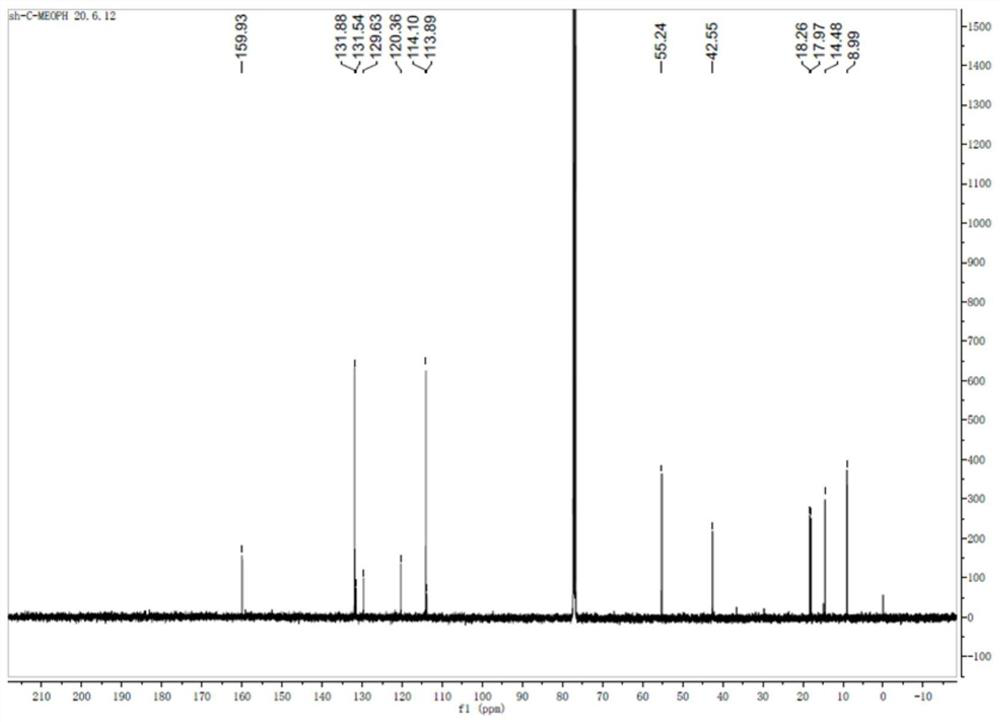
[0028] The sulfose ligand in the auranofin structure will be replaced with selenium-containing imidazole nitrogen heterocyclic carbene selenium, the reactivity of Au-Se is higher than that of Au-S bonds, and the above compound H7 and compound H8 are obtained by chemical synthesis methods, and Its structure was confirmed by NMR analysis.
[0029] Synthetic steps of H7 and H8: using p-substituted benzaldehyde as starting material, under the catalysis of thiamine hydrochloride, a benzoin condensation reaction occurs, the obtained p-substituted benzoin compound is refluxed with formamide, and the ring is closed to obtain 4, The 5-diaryl substituted imidazole compound, and then reacted with bromoethane to generate a single N-ethyl substituted 4,5-diaryl substituted imidazole compound, and finally reacted with the correspon...
Embodiment 2
[0044] Example 2: In vitro antibacterial activity of nitrogen heterocyclic carbene selenium-gold compounds H7 and H8 against carbapenem-resistant Acinetobacter baumannii
[0045] Compounds H7 and H8 were tested for antibacterial activity and minimum inhibitory concentration, referring to CLSI (American Clinical Standards Institute) M07-A9 microbroth dilution method. The bacterial solution with 600nm OD value of 1 was diluted to 0.5 McFarland turbidity with a McFarland turbidimetric tube, and continued to dilute 1000 times to obtain the experimental bacterial solution, which was set aside. Continue to analyze the antibacterial activity, use 96-well plate, add monomer compound in the 4th to 7th column, the final concentration of the compound is 1μM, 2μM, 5μM, 10μM. A negative control group was set up in columns 2-3, and LB medium was added to the remaining wells as a blank control, incubated at 37°C for 24 hours, and the lowest monomeric compound concentration at which the bacte...
Embodiment 3
[0050] Example 3: In vivo anti-infection evaluation of compound H7 and compound H8
[0051] Through the determination of the minimum inhibitory concentration, compounds H7 and H8 both showed good antibacterial activity. Therefore, we systematically evaluated the in vivo anti-infection activities of H7 and H8 at the animal level in mice, and the specific methods were as follows:
[0052] 22-25g Balb / c mice were fed adaptively for 1 week, and surgical scissors were used to cut a 1.2*1.2cm wound on the back skin of the mouse, and 2*10 6 200 μL of CFU / ml bacterial solution was used to establish a wound infection model, and they were randomly divided into five groups, with 6 animals in each group. After 24 hours of infection, the control group was given external application of normal saline to the wound; H 2 o 2 Group (positive control) was given external application of 10mM concentration of hydrogen peroxide wound; AF group (positive control) was given external application of au...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com