Fully-conjugated spiral fused ring trapezoidal organic molecule as well as preparation method and application thereof
A technology of organic molecules and fused rings, applied in organic chemistry, chemical instruments and methods, preparation of amino compounds from amines, etc., can solve the problems of lack of organic nonlinear optical material systems, poor photothermal stability, lack of species, etc. Effects of laser thermal stability, luminescence stability, and high laser gain characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1: Realize the synthesis of precursor 1, 2, 3
[0053] We start from simple raw materials to synthesize target precursors 1, 2, 3, such as the reaction scheme Figure 1 . Among them, step (I) realizes Friedel-Crafts acylation and Friedel-Crafts alkylation reaction through terebromophthalic acid to obtain important monomer 4; step (II) carries out Suzuki coupling reaction through important monomer 4 and triphenylamine borate Precursor 1 is obtained; Step (III) realizes Ullmann's Ullman under the condition of 100°C by using raw materials 2,7-dibromomethylfluorene and diphenylamine under the action of sodium tert-butoxide and L-proline and dioxane as solvent. Coupling reaction obtains monomer 5; Step (IV) obtains its borate monomer 6 by monomer 5 through diboronic acid pinacol ester, divalent palladium catalysis, dioxane solvent conditions; Step (V) Carry out Suzuki coupling reaction by monomer 6 and important monomer 4 to obtain precursor 2; Step (VI) obtain ...
Embodiment 2
[0056] Embodiment 2: the synthesis of compound A
[0057]
[0058] 【Reaction route Figure II 】Such as Figure 19 shown.
[0059]
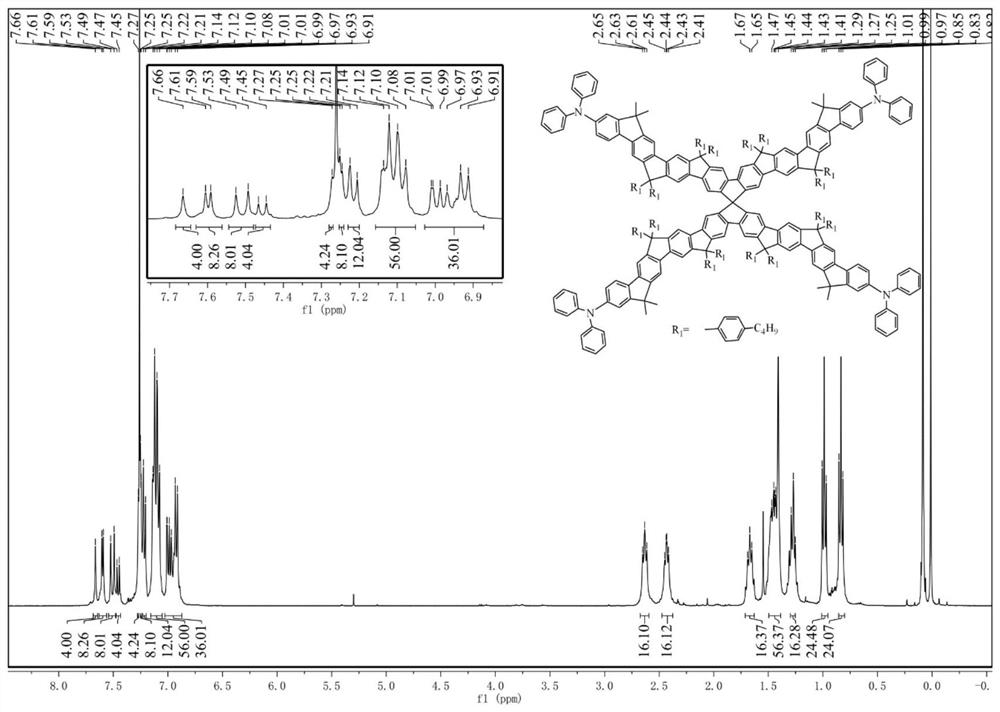
[0060] According to the reaction scheme, tetrabromospirofluorene is obtained through diboronic acid pinacol ester, divalent palladium catalysis, and dioxane solvent to obtain spirofluorene tetraborate monomer; spirofluorene tetraborate, precursor 1 The four-arm spirofluorene precursor was obtained by Suzuki coupling reaction; the lithiation of bromobutylbenzene was realized by butyl lithium, and the lithium butylbenzene attacked the carbonyl active site, and finally the cyclization process was realized by boron trifluoride ether. The specific experimental process is as follows:
[0061] Step i: Under nitrogen, 2,2',7,7'-tetrabromospirofluorene (1.26g, 2.0mmol), biboronic acid pinacol ester (3.05 g, 12.0mmol), potassium acetate (1.88g , 18.0mmol) and 1,4-dioxane (30mL) were added to a 100mL reaction flask, followed by the addition of Pd (...
Embodiment 3
[0065] Embodiment 3: the synthesis of compound B
[0066]
[0067] 【Reaction route Figure three 】Such as Figure 20 shown.
[0068]
[0069] The synthesis method of compound B is similar to the synthesis method of compound A.
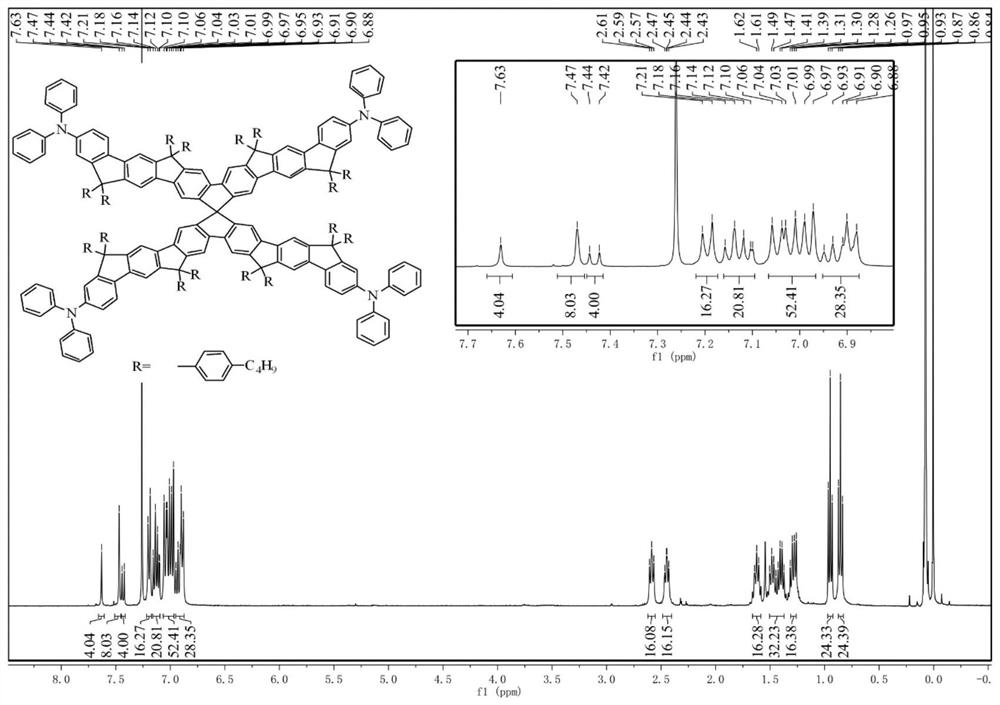
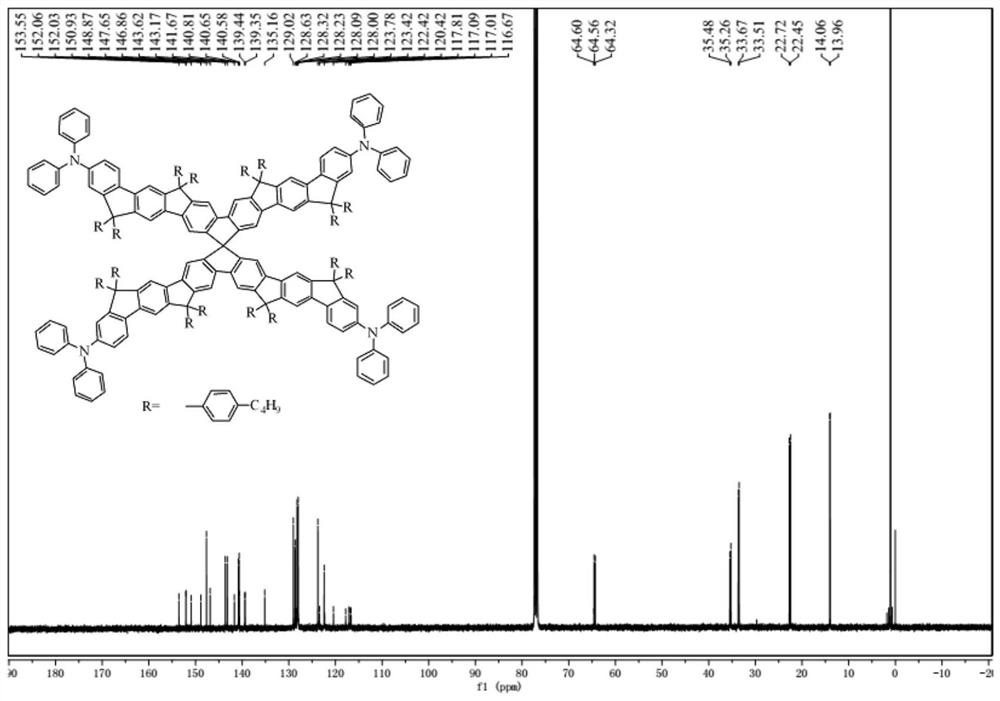
[0070] 1 H NMR (400MHz, CDCl 3 ,δ):7.66(s,4H),7.60(d,J=5.5Hz,8H),7.51(d,J=12.9Hz,8H),7.46(d,J=8.3Hz,4H),7.27(s ,4H),7.25(d,J=2.5Hz,8H),7.21(d,J=7.6Hz,12H),7.11(d,J=16.1Hz,56H),7.03–6.87(m,36H),2.68 –2.59(m,16H),2.43(d,J=8.2Hz,16H),1.72–1.63(m,16H),1.50–1.39(m,56H),1.27(t,J=7.2Hz,16H), 0.99(t,J=7.3Hz,24H),0.83(t,J=7.3Hz,24H). 13 C NMR (100MHz, CDCl 3 ,δ):155.44,152.98,152.17,151.94,151.76,151.72,148.89,148.22,148.00,146.99,143.74,143.65,141.80,140.84,140.71,140.61,140.07,139.80,139.03,138.49,134.54,129.17,128.75, 128.35,128.16,128.06,123.89,123.55,122.47,120.51,118.86,117.77,117.30,117.15,116.80,114.02,64.63, 64.38,64.35,46.48,35.54,35.30,33.71,33.52,27.35,22.76,22.51,14.09, 13.95. MALDI-TOF MS(m / z): calcd for C 325 h 308 N 4 ,exact m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com