Method for recovering arsenic and valuable metals from arsenic-containing soot
A valuable metal, arsenic recovery technology, applied in chemical instruments and methods, arsenic oxide/arsenic hydroxide/oxyacid arsenic, arsenic compounds, etc. Secondary pollution and other problems, to achieve the effect of improving the recovery rate and improving the reduction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
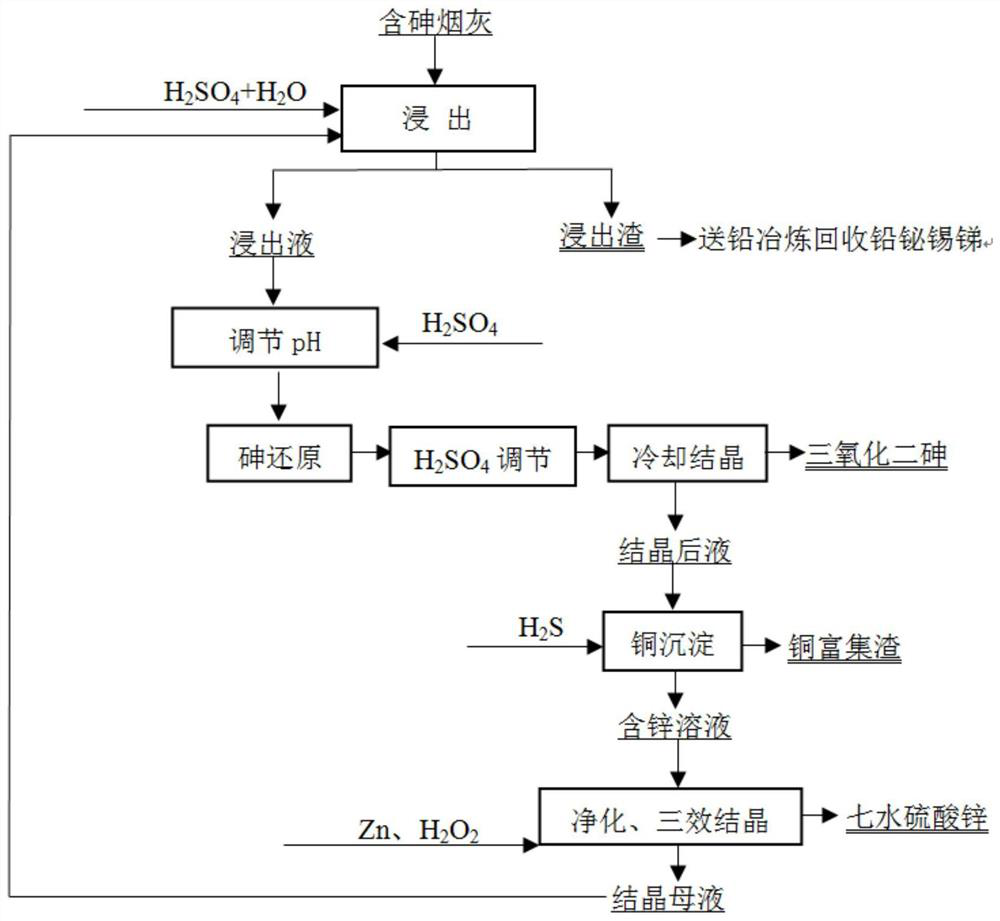
[0040] (1) Take 100g of arsenic-containing copper soot, including 15.32% arsenic, 5.12% copper, 10.62% zinc, 14.86% lead, 3.21% bismuth, 1.26% antimony, and 1.53% tin.
[0041] (2) Add 10mL of sulfuric acid, 30mL of hydrogen peroxide, and 260mL of water to the soot, and leach for 3 hours at a leaching temperature of 85°C. After the leaching is complete, filter to obtain the leach residue and leach solution. Lead, bismuth, antimony, and tin are all left in the leach residue , The recoveries of lead, bismuth, antimony, and tin were 99.32%, 96.13%, 90.16%, and 95.32%, respectively.
[0042] (2) After adding 20mL of sulfuric acid to the leaching solution, 30g of sulfur dioxide (SO 2 The feed rate is 50mL / min), after the addition of sulfur dioxide, continue to stir and react for 0.5h, then add 28mL of sulfuric acid, and put the solution into the cooling room for stirring and cooling at 5°C. After cooling for 1 hour, diarsenic trioxide and crystallized solution were obtained by fil...
Embodiment 2
[0046] (1) Take 100g of arsenic-containing lead soot, including 20.53% arsenic, 0.53% copper, 12.33% zinc, 18.06% lead, 2.19% bismuth, 2.56% antimony and 2.01% tin.
[0047] (2) Add 9mL of sulfuric acid, 35mL of hydrogen peroxide, and 256mL of water to the soot, and leach for 3 hours at a leaching temperature of 85°C. After the leaching is complete, filter to obtain the leach residue and leach solution. Lead, bismuth, antimony, and tin are all left in the leach residue , The recoveries of lead, bismuth, antimony, and tin were 99.01%, 95.32%, 89.33%, and 92.21%, respectively.
[0048] (2) After adding 22mL of sulfuric acid to the leaching solution, 45g of sulfur dioxide (SO 2 The feed rate is 50mL / min), after the addition of sulfur dioxide, continue to stir and react for 0.5h, then add 30mL of sulfuric acid, and put the solution into the cooling room to stir and cool under the condition of 3°C. After cooling for 1 hour, filter to obtain diarsenic trioxide and crystallized liqu...
Embodiment 3
[0052] (1) Take 100g of arsenic-containing tin soot, including 9.26% arsenic, 6.11% copper, 8.16% zinc, 15.33% lead, 7.83% bismuth, 3.62% antimony, and 9.13% tin.
[0053] (2) Add 12mL of sulfuric acid, 35mL of hydrogen peroxide, and 253mL of water to the soot, and leach for 3 hours at a leaching temperature of 80°C. After the leaching is complete, filter to obtain the leach residue and leach solution. Lead, bismuth, antimony, and tin are all left in the leach residue , The recoveries of lead, bismuth, antimony, and tin were 99.52%, 96.13%, 92.16%, and 93.16%, respectively.
[0054] (2) After adding 20mL of sulfuric acid to the leaching solution, 25g of sulfur dioxide (SO 2 The feed rate is 50mL / min), after the addition of sulfur dioxide, continue to stir and react for 0.5h, then add 30mL of sulfuric acid, and put the solution into the cooling room to stir and cool under the condition of 3°C. After cooling for 1 hour, filter to obtain diarsenic trioxide and crystallized solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com