Application of mass spectrum technology to detection of molecular level of PSM-E in urine in preparation of product for early diagnosis of prostate cancer
A PSM-E, prostate specific technology, applied in the field of clinical examination and diagnosis, to achieve the effect of strong versatility, reducing errors and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Synthesis of standard working fluids in Example 1 PSM-E
[0035] First, experimental method
[0036] 1, Synthesis of PSM-E Characteristic Peptide Standards
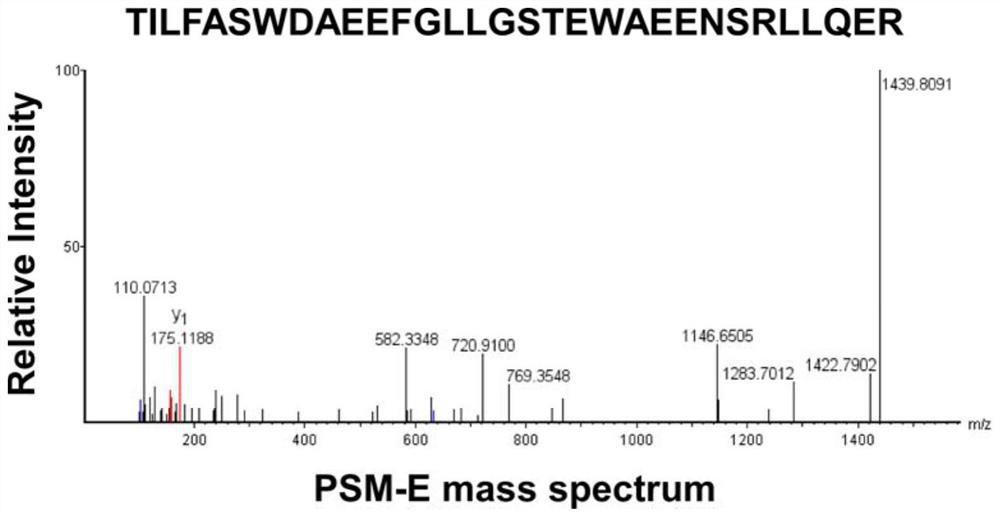
[0037] The PSM-E feature peptide standard is synthesized via Hangzhou Zhongopeptide Co., Ltd., its amino acid sequence is: TilfaswdaeefllgstewaeenSrlllll (SEQ ID NO: 2); 5 mg; purity 99.5%.
[0038] 2, preparation for PSM-E characteristics peptide standard
[0039] Use DDH 2 O Dilute the BSA standard to 1 ng / μL. Set 8 concentration gradient protein labels: Standard product is added to the standard pores of 96-well cultural sheets by 0, 1, 2, 4, 8, 10, 12, 14, 16, 18, and 20 μL, less than 20 μl Hole with ultrapure water to 20 μL.
[0040] Table 1 Standard product plus sample table
[0041]
Embodiment 2
[0042] Detection of Example 2 wherein the content of the peptide in urine embodiment PSM-E
[0043] A urine specimen proteolysis
[0044] A method as follows:
[0045] (1) The urine samples were transferred to the corresponding 10KDa ultrafiltration tubes, 12,000g 20min centrifugation;
[0046] (2) per each ultrafiltration plus 200μl Buffer1 (8M urea / 100mM Tris-HCl, pH 8.5), sufficiently soluble denatured protein;
[0047] (3) Add 20μl DTT (100mM) solution, 37 ℃ for 2h reduce disulfide bonds;
[0048] (4) Add 20μl IAA (500mM) was dark at room temperature the reaction 15min;
[0049] (5) the protein solution was centrifuged 12,000g reductive alkylation after 20min, the bottom of the collection tube was discarded. Add 200uL Buffer1 centrifugation was repeated twice;
[0050] (6) Add 200μl Buffer2 (8M urea / 100mM Tris-HCl, pH 8.0), 12,000g centrifugation 20min, the bottom of the collection tube was discarded, repeat 1;
[0051] (7) Add 200μl solution of ammonium bicarbonate (25 mM...
Embodiment 3
[0065] Detecting a feature peptide of Example 3 embodiment urine PSM-E
[0066] First, experimental method
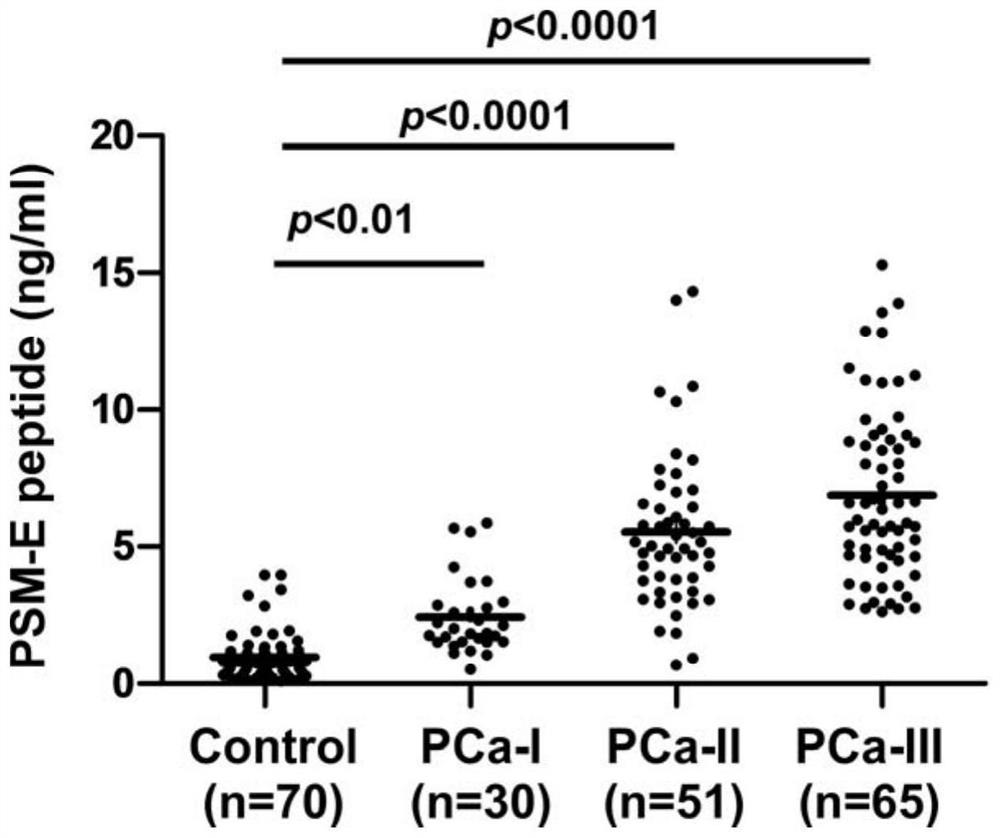
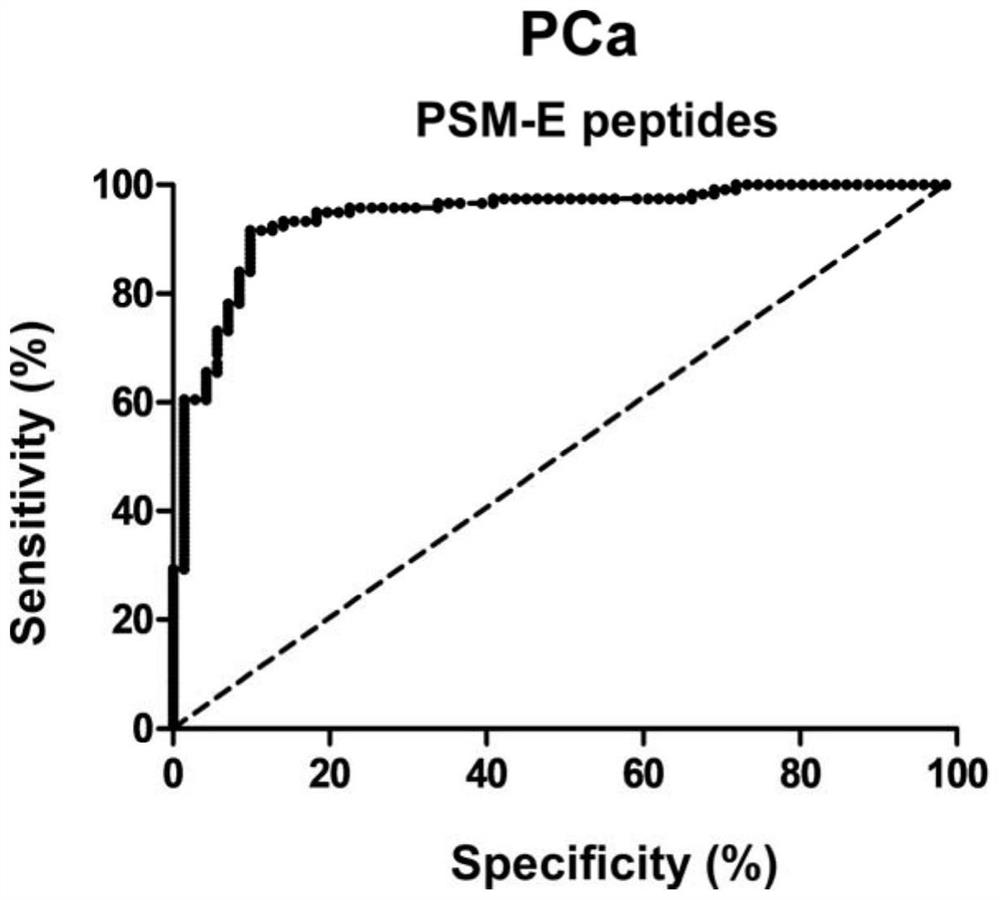
[0067] Enrolled 132 subjects, differences among groups of sex, age, blood pressure, smoking, drug use and other leavened dough were not statistically significant, comparable. Collected 70 healthy persons group (Control), the patient 30 Example I prostate carcinoma (PCa-I), 51 Example II prostate carcinoma (PCa-II) and 65 stage III prostatic carcinoma (PCa-III) the method of Example 3 sera, using, the content of each group detected in urine specimens PSM-E characteristics peptide.
[0068] Second, the experiment
[0069] like figure 2 with 3 Shown, PSM-E wherein peptide (SEQ ID NO: 2) in the urine for the concentration of each group were 0.9831 ± 0.8322ng / ml, 2.4430 ± 1.3780ng / ml, 5.5170 ± 2.836ng / ml, 6.8740 ± 3.1350 ng / ml. Which, compared with the healthy group, in urine samples I-III prostate patients PSM-E features peptide were significantly increased, and the dif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com