Biodegradable complex microbial inoculant for aureomycin fungus residue waste and compost high-temperature pre-fermentation method of biodegradable complex microbial inoculant
A compound bacterial agent and biodegradation technology, which is applied in the direction of microorganism-based methods, biochemical equipment and methods, and fertilizers made from biological waste, can solve the problems of weak adaptability of aureomycin fermentation slag, and achieve the goal of shortening the time-consuming Harmful time, enhance vitality, and realize the effect of resource utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1-test bacterial strain is to aureomycin tolerance
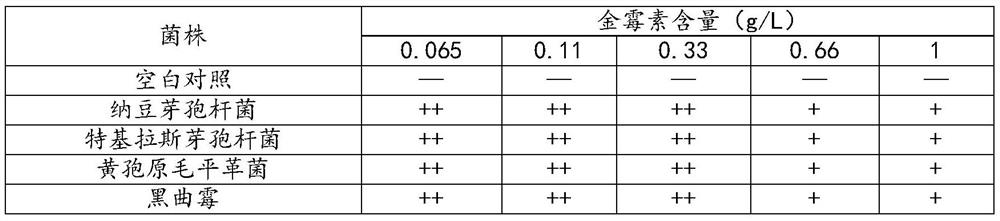
[0034] The strains of Bacillus natto, Bacillus tequilas, Bacillus tequilas, Phanerochaete chrysosporium, and Aspergillus niger were respectively inoculated into inorganic salt medium with different concentrations of aureomycin as carbon source, After shaking the flask at 30°C and 180rpm for 48 hours, observe the growth of the strain. The test results are shown in Table 1:
[0035] The growth situation of table 1 bacterial strain in the inorganic salt medium containing different concentrations of aureomycin
[0036]
[0037] Note: — indicates sterile growth, ++ indicates good growth, + indicates growth or slight inhibition
[0038] Among them, the composition of the inorganic salt medium is: NaCl 0.02%, dipotassium hydrogen phosphate 0.04%, potassium dihydrogen phosphate 0.04%, NH 4 NO 3 0.2%, magnesium sulfate 0.01%, manganese sulfate 0.001%, ferrous sulfate 0.001%, calcium chloride 0.01%, after ster...
Embodiment 2
[0040] The preparation of embodiment 2-bacterial strain bacterial powder
[0041] A. Preparation of Bacillus natto bacterium powder: Inoculate Bacillus natto into LB culture medium, culture in shake flask at 37°C and 180rpm for 24h, then centrifuge and dehydrate the obtained culture, and then freeze-dry in a vacuum to obtain each bacterial strain Freeze-dried powder, finally mix the freeze-dried powder with corn starch to make 1ⅹ10 8 cfu / g of bacteria powder;
[0042] B. Preparation of Bacillus tequilas bacteria powder: adopt the same preparation method as above-mentioned Bacillus natto bacteria powder to make 1ⅹ10 8 cfu / g of bacteria powder;
[0043] C. Preparation of Aspergillus niger powder: inoculate Aspergillus niger into solid bran medium (mixing bran and water at a ratio of 1:1) and culture at 37°C for 120 hours, after-cooking at room temperature for 48 hours, during which the koji is turned once a day; The cultured koji is mixed with bran, dried at 45-55°C for 24 ho...
Embodiment 3
[0045] Degradation test of bacterial residue solid waste after the extraction of aureomycin by the bacterial strain of embodiment 3
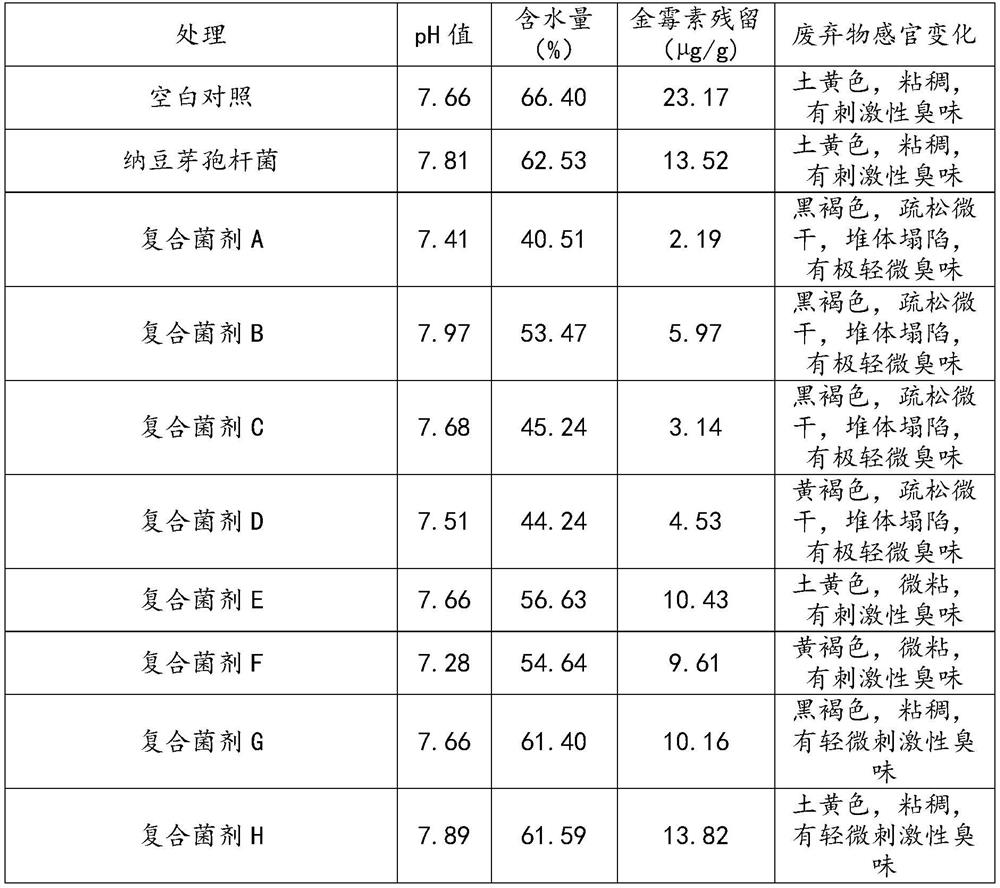
[0046] The bacteria powder prepared in Example 2 was respectively added to the solid waste of the bacteria residue after the extraction of aureomycin to ferment and test the degradation effect. The specific operation method is as follows:
[0047] Take aureomycin fermented and extracted fungus residue solid waste, water content is about 45%, pH is between 7-9, crushed to below 5mm, then add a certain amount of water to adjust the water content of fungus residue to about 60% , adjust the pH to about 7, pack in 70g / 500mL Erlenmeyer flasks, and aseptically sterilize. Then add 1 mg / g of sterile aureomycin in each conical flask, mix well, add the bacterial strain prepared in Example 2 with 5% inoculation amount, put in 37 ℃ of static culture 5d put bottle, simultaneously with The fermentation substrate without strain inoculation was used as a contro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com