Preparation method of calcium glycinate complex
A technology of calcium glycinate and complexes, which is applied in the field of preparation of calcium glycinate complexes, can solve problems affecting the purity of calcium glycinate complex products, complicated process steps, and large energy loss, so as to reduce resource input and improve product quality. Purity, the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
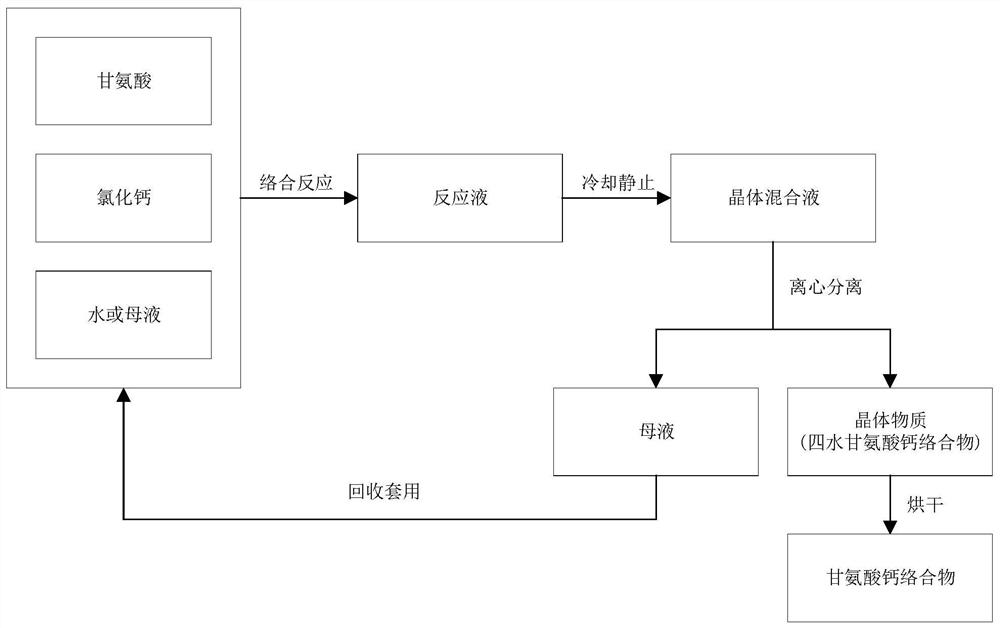
[0030] see figure 1 , a preparation method of calcium glycinate complex, comprising the steps:
[0031] Step S100, put glycine and anhydrous calcium chloride in a reaction kettle with a molar ratio of 2:0.8-1.5, and dissolve them in a solvent, then stir and mix, the solvent is water or mother liquor, heated to 60-100° C. After the complexation reaction process, a reaction solution is obtained. The pH value of the reaction solution obtained by dissolving an appropriate proportion of glycine and anhydrous calcium chloride in a solvent is in the range of 4 to 5, which can meet the requirements of complexation reaction.
[0032] According to the complexation reaction process, the reaction formula of the complexation reaction includes the following:
[0033] Ca 2+ +6H 2 O+ heat → [Ca(H 2 O) 6 ] 2+
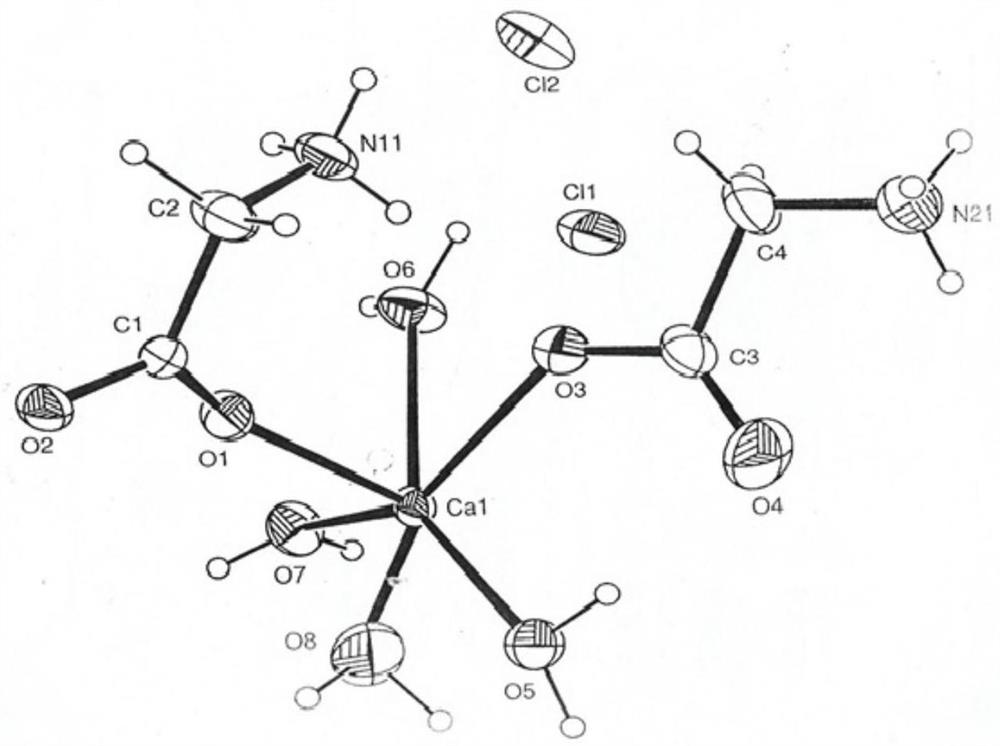
[0034] [Ca(H 2 O) 6 ] 2+ +2C 2 H 5 NO 2 →[Ca(C 2 H 5 NO 2 ) 2 ·4H 2 O] 2+ +2H 2 O
[0035] In step S200, the reaction solution is cooled to stand still, and the pr...
Embodiment 1
[0058] Get food-grade content and be 98.5% glycine 1000kg, get food-grade content 98% anhydrous calcium chloride 740kg, drop into slowly to add 1500kg (1.5m 3 ) water in a reaction kettle, stirring and mixing, while gradually heating the reaction solution until the reaction temperature is 90°C, so that the solid matter in the reaction solution is completely dissolved, and the reaction is completed after maintaining the reaction temperature for 2 hours, and the reaction solution with a pH value of 4.8 is obtained.
[0059] Pour the reaction solution into the crystallization tank, cool down with circulating cooling water, and at the same time stir slowly to balance the temperature of each part of the reaction solution. When the temperature of the reaction solution is close to room temperature (25°C), sprinkle a small amount of white powder of calcium glycinate complex as a crystal. species to facilitate crystallization. A large number of crystals were precipitated in the reactio...
Embodiment 2
[0063] Get 500kg of glycine with a food grade content of 98.5%, and 370kg of anhydrous calcium chloride with a food grade content of 98%. 3 In the reaction kettle of the mother liquor, stir and mix, and gradually heat the reaction solution until the reaction temperature is 90° C. to completely dissolve the solid matter in the reaction solution, keep the reaction temperature for 2 hours, and the reaction ends to obtain a reaction solution with a pH value of 4.76.
[0064] Pour the reaction solution into the crystallization tank, cool it down with circulating cooling water, and at the same time stir slowly to balance the temperature of each part of the reaction solution. When the temperature of the reaction solution is close to room temperature (26°C), sprinkle a small amount of white powder of calcium glycinate complex as a crystal. species to facilitate crystallization. A large number of crystals were precipitated in the reaction solution, and its temperature was very close to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com