Method for measuring calcium ions after dissolution of dolomite
A determination method, calcium ion technology, applied in the field of mineral processing, can solve problems such as relatively high requirements in the operation process, complicated process, and danger
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
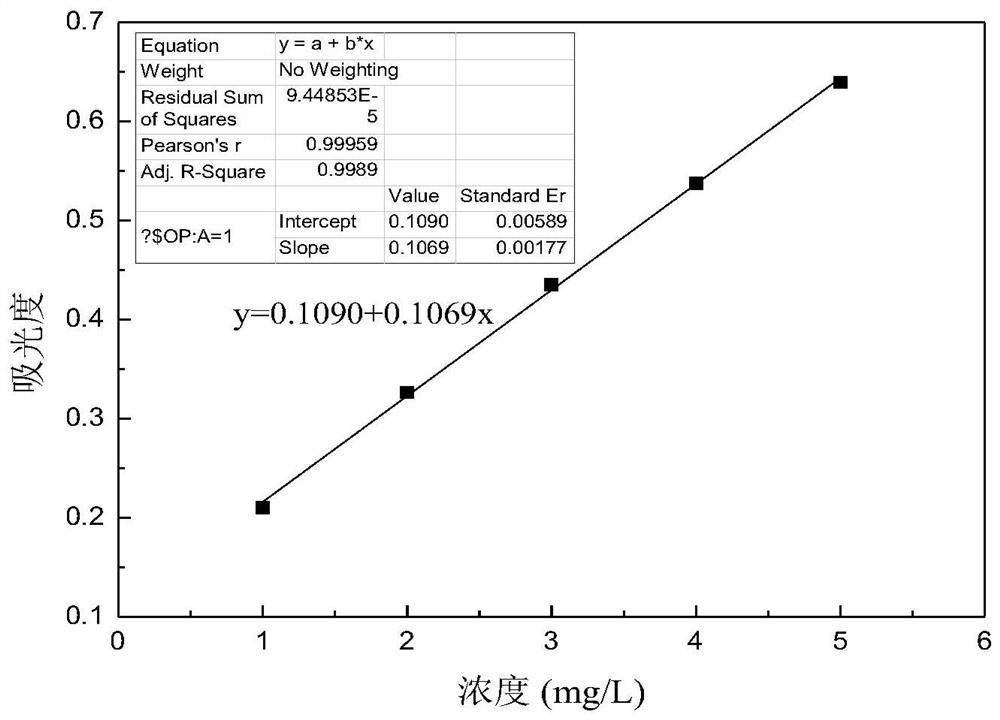
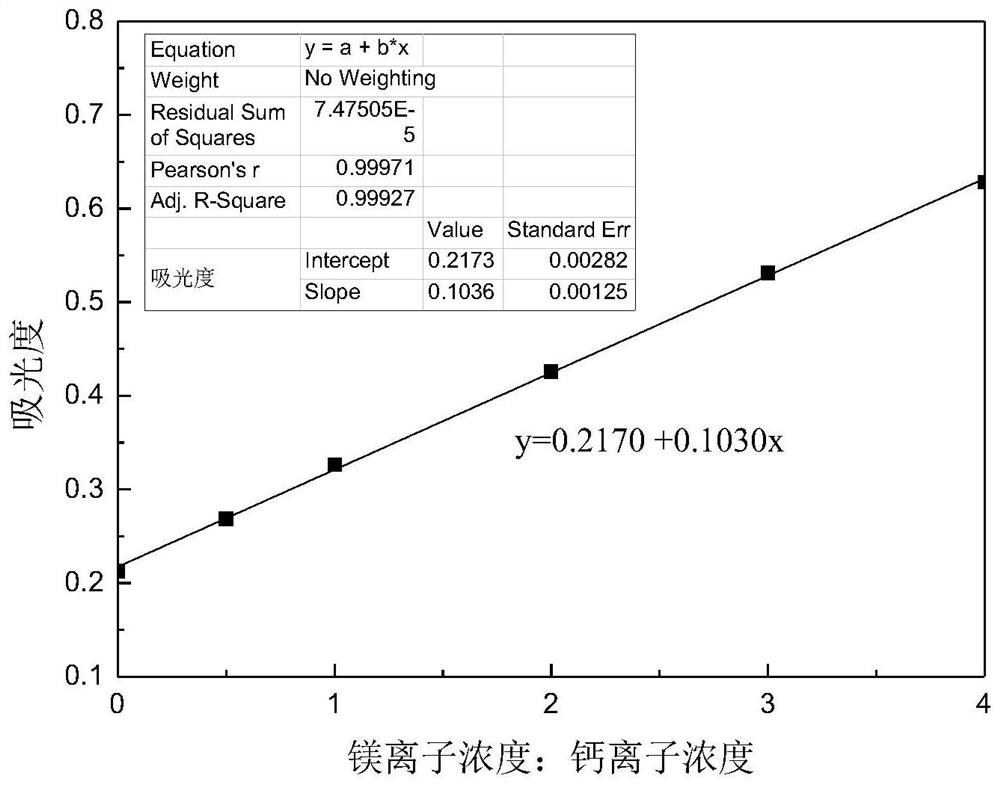
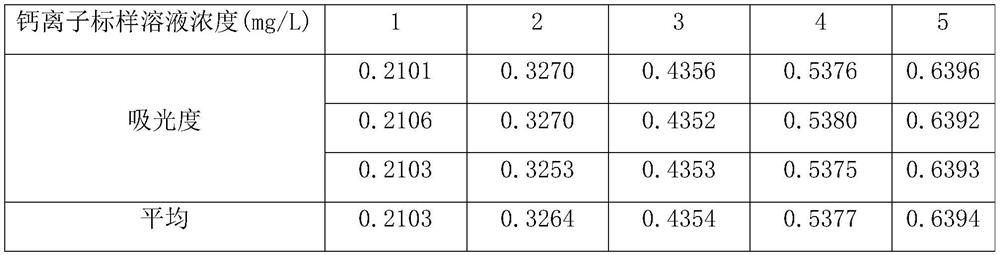
[0027] A kind of assay method of calcium ion after dolomite dissolves, specifically comprises the steps:
[0028] 1) Take a glass container (50ml beaker or Erlenmeyer flask), and configure 0.005mol / L acid chrome blue K color developer, acid chrome blue K can develop color simultaneously with calcium and magnesium ions dissolved in dolomite;
[0029] 2) Take a glass container (250ml beaker or Erlenmeyer flask) and configure an ammonia-ammonium chloride buffer with pH=10. Under the ammonia-ammonium chloride buffer system, the calcium and magnesium ions dissolved in dolomite and acid chromium blue K is rose red and forms a stable complex, while other ions (such as iron ions, etc.) in the dolomite dissolution system cannot form stable complexes with these two agents, thus having little influence on the determination of calcium ions;
[0030] 3) Take 6 25ml volumetric flasks and add 0 (for zeroing of UV-visible spectrophotometer) and 0.125ml, 0.25ml, 0.375ml, 0.50ml, 0.625ml of 0.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com