Method for preparing aldehyde/ketone by breaking C-C bond
A bond breaking and C-C technology, which is applied in the field of C-C bond breaking to prepare aldehydes/ketones, can solve the problems of difficult large-scale application in industrial production, industrial application restrictions, and high price.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
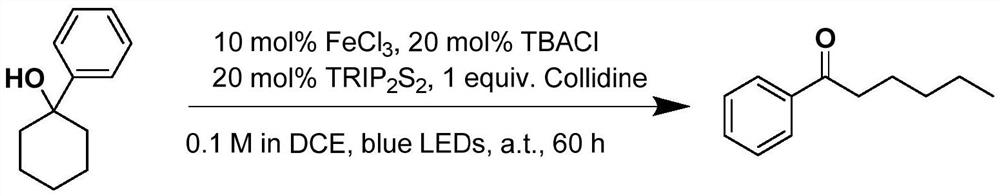
[0051] The present embodiment has prepared a kind of phenhexanone, and concrete process is:
[0052]
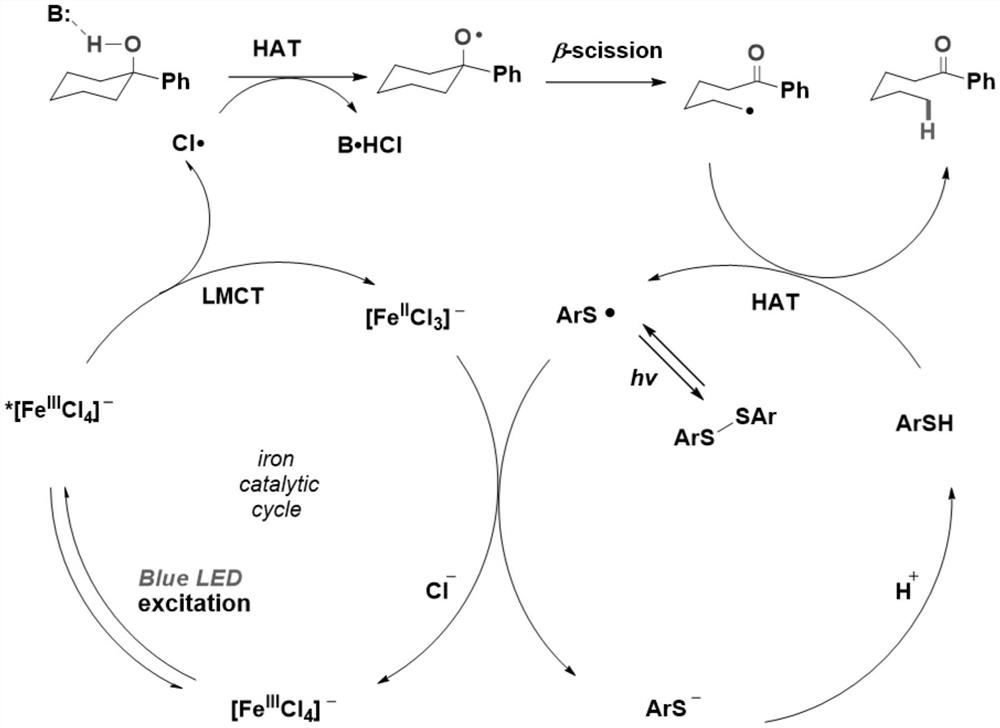
[0053] At room temperature, 1-phenylcyclohexanol (0.2mmol, 1equiv), ferric chloride (0.02mmol, 0.1equiv), tetrabutylammonium chloride (0.04mmol, 0.2equiv), bis(2,4 , 6-triisopropylphenyl) disulfide (0.04mmol, 0.2equiv), 2,4,6-collidine (0.2mmol, 1equiv) and 2mL dichloroethane were added to the reaction tube, and the replacement Protective gas three times, stirring reaction 60h under the illumination of 450nm blue light, after monitoring the reaction by thin-layer chromatography, the reaction mixture was cooled, then added ethyl acetate to filter, then spin off the solvent, and obtain the product after column chromatography separation (washing Removal agent: petroleum ether: ethyl acetate = 20:1), the product is a yellow liquid with a yield of 81%.
[0054] The data of the proton nuclear magnetic resonance spectrum of gained product are as follows:
[0055] 1 H NMR (400M...
Embodiment 2
[0060] The present embodiment has prepared a kind of phenhexanone, and concrete process is:
[0061]
[0062] At room temperature, 1-phenylcyclohexanol (0.2mmol, 1equiv), iron trichloride (0.02mmol, 0.1equiv), tetrabutylammonium chloride (0.04mmol, 0.2equiv), diphenyl disulfide Add ether (0.04mmol, 0.2equiv), 2,4,6-collidine (0.2mmol, 1equiv) and 2mL dichloroethane into the reaction tube, replace the protective gas three times, and stir the reaction under the light of 450nm blue light After 24h, after the end of the reaction was monitored by thin layer chromatography, the reaction mixture was cooled, then ethyl acetate was added to filter, then the solvent was spun off, and the product was obtained after column chromatography separation (eluent: petroleum ether: ethyl acetate=20: 1), the product is a yellow liquid, and the yield is 25%.
[0063] The data of the proton nuclear magnetic resonance spectrum of gained product are as follows:
[0064] 1 H NMR (400MHz, Chlorofo...
Embodiment 3
[0066] The present embodiment has prepared a kind of 4-trifluoromethyl acetohexanone, and concrete process is:
[0067]
[0068] At room temperature, 1-(4-trifluoromethylphenyl)cyclohexanol (0.2mmol, 1equiv), ferric chloride (0.02mmol, 0.1equiv), tetrabutylammonium chloride (0.04mmol, 0.2 equiv), bis(2,4,6-triisopropylphenyl) disulfide (0.04mmol, 0.2equiv), 2,4,6-collidine (0.2mmol, 1equiv) and 2mL dichloroethyl Add alkane into the reaction tube, replace the protective gas three times, stir the reaction under the light of 450nm blue light for 60h, monitor the end of the reaction by thin layer chromatography, cool the reaction mixture, then add ethyl acetate to filter, then spin off the solvent, pass through the column The product was obtained after chromatographic separation (eluent: petroleum ether: ethyl acetate = 20:1), and the product was a yellow liquid with a yield of 86%.
[0069] The data of the proton nuclear magnetic resonance spectrum of gained product are as fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com