Clarithromycin spherical crystal as well as preparation method and application thereof
A technology of spherical crystals and clarithromycin, which is applied in the field of medicine, can solve the problems of uneven crystal particle size distribution, poor fragmentation and friability, and increased production costs, and achieve simple operation, improved fluidity and compressibility, and spherical The effect of compact and round crystal particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
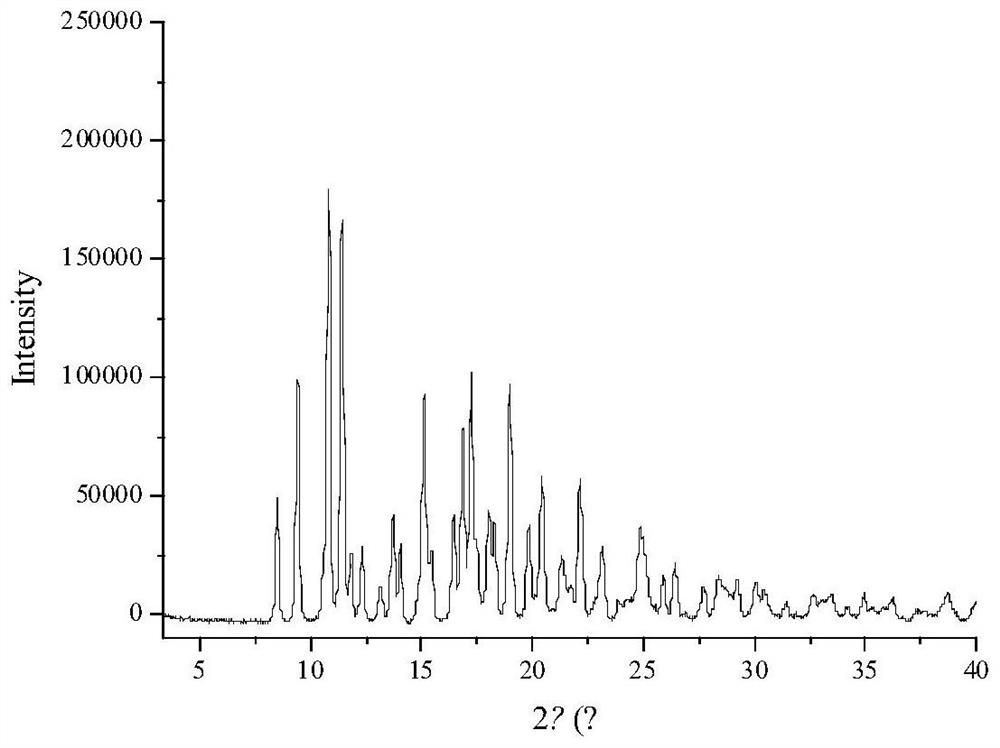
[0045] Preparation of clarithromycin spherical crystals
[0046] Take about 0.2 g of clarithromycin raw material, weigh it accurately, put it in a beaker, add 20 mL of good solvent methanol, and dissolve it by ultrasonic; take about 0.03 g of hydroxypropyl cellulose, and slowly add it to the container under stirring conditions. In a beaker with 100 mL of water, stir continuously to dissolve it completely, and place it in a crystallization container; add the drug solution and place it immediately for 25 o Shake fully at 250 rpm in a constant temperature shaking incubator. After 40 min, the resulting suspension was suction-filtered, and the solid product was o C. Dry under reduced pressure in a vacuum oven for 24 h, and the resulting dried solid particles are the product.
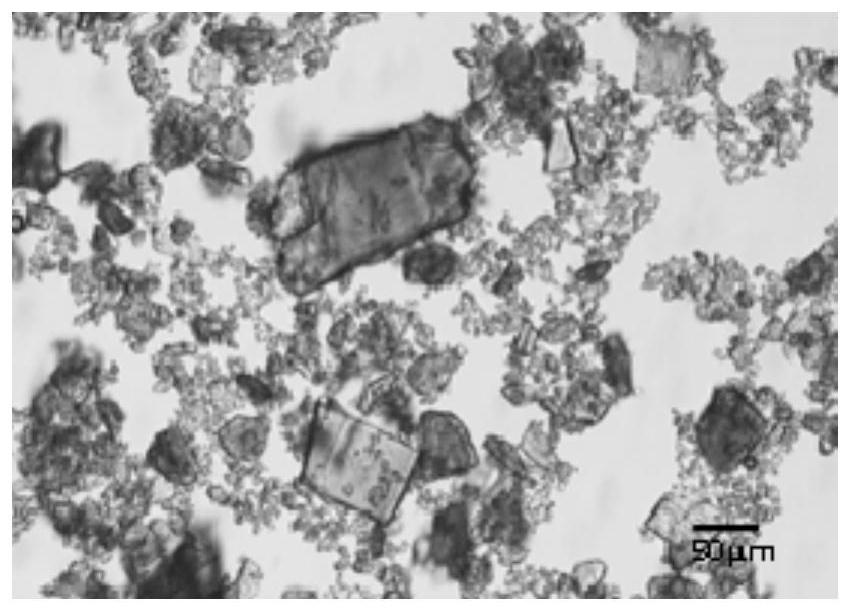
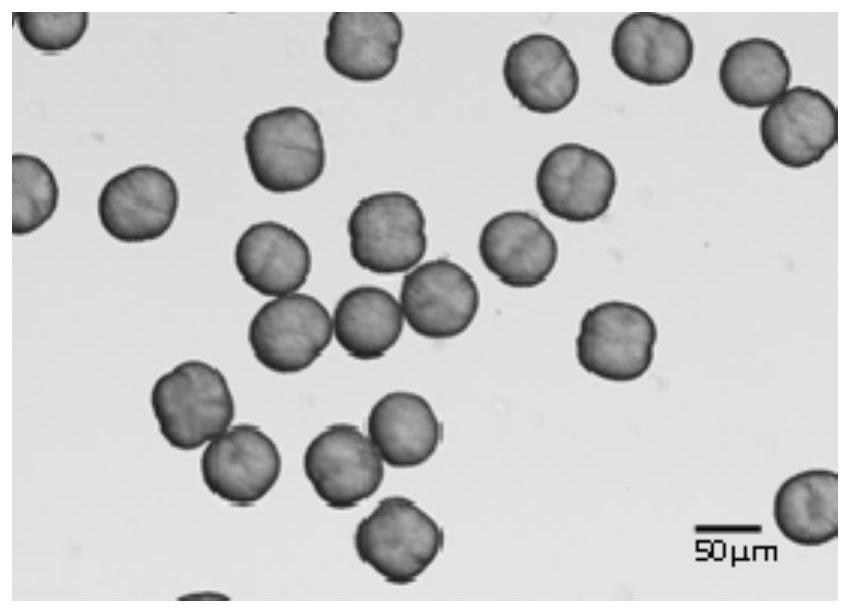
[0047] The appearance of the product is spherical, the Carr index is 15.44%, the angle of repose is 30.38°, d 90 is 55 μm.
Embodiment 2
[0049] Preparation of clarithromycin spherical crystals
[0050] Take about 0.1 g of clarithromycin raw material, weigh it accurately, put it in a beaker, add 20 mL of good solvent ethanol, and dissolve it by ultrasonic; take about 2 g of hydroxypropyl methylcellulose, and slowly add it under the condition of stirring into a beaker filled with 40 mL of water, stir continuously to dissolve it completely, and place it in a crystallization vessel; add the drug solution and place it immediately for 30 o Shake thoroughly at 100 rpm in a constant temperature shaking incubator. After 90 min, the resulting suspension was suction-filtered, and the solid product was o C. Dry under reduced pressure in a vacuum oven for 48 h, and the resulting dried solid particles are the product.
[0051] The appearance of the product is spherical, the Carr index is 20.35%, the angle of repose is 39.68°, d 90 is 60 μm.
Embodiment 3
[0053] Preparation of clarithromycin spherical crystals
[0054] Take about 1 g of clarithromycin raw material, accurately weigh it, put it in a beaker, add 20 mL of good solvent ethyl acetate, and ultrasonically dissolve it; take about 6 g of methylcellulose, and slowly add it to In a beaker with 200 mL of water, stir continuously to dissolve it completely, and place it in a crystallization container; add the drug solution and place it immediately for 20 o Shake fully at 300 rpm in a constant temperature shaking incubator. After 50 min, the resulting suspension was suction-filtered, and the solid product was o C. Dry under reduced pressure in a vacuum oven for 24 h, and the resulting dried solid particles are the product.
[0055] The appearance of the product is spherical, the Carr index is 19.76%, the angle of repose is 37.65°, d 90 is 58 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle of repose | aaaaa | aaaaa |
| angle of repose | aaaaa | aaaaa |
| angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com