7beta-HSDH enzyme mutant as well as coding gene and application thereof
A technology for encoding genes and mutants, which is applied to 7β-HSDH enzyme mutants and their encoding genes and application fields, can solve the problems of unsatisfactory hydroxysteroid dehydrogenase activity and difficulty in realizing industrialized production, etc., and achieves broad industrial application prospects, The effect of reducing the amount of enzyme used and reducing the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The construction of embodiment one prokaryotic expression system
[0063] The 7β-HSDH gene fragment was synthesized by Changzhou Jiyu Biotechnology Co., Ltd., and recombined into the PUC57 vector. After double digestion with restriction endonucleases NdeI and HindIII (purchased from New England Biolabs, NEB) at 37°C for 4 hours, they were separated by 1% agarose gel electrophoresis and recovered by gel cutting (the gel recovery kit was purchased from Tiangen Biochemical Technology (Beijing) Co., Ltd.). Subsequently, it was ligated with the expression vector pET21a(+) (Novagen Company) that had also undergone double enzyme digestion, and placed in a low-temperature ligator under the action of T4 DNA ligase (purchased from Takara Company) overnight. The connection solution was used to transform DH5a competent cells (purchased from Tiangen Biochemical Technology (Beijing) Co., Ltd.), and colony PCR screening and sequencing verification were performed to obtain the positiv...
Embodiment 2
[0066] Shake flask fermentation of embodiment two enzymes prepares enzyme freeze-dried powder
[0067] The expression strain 7β-HSDH-pET21a(+) / BL21(DE3) constructed above was added to 5mL LB liquid medium [10g / L tryptone (OXIOD), 5g / L yeast powder (OXIOD), 10g / L sodium chloride (Sinopharm Reagent)】, shake culture overnight at 37°C and 200rpm, inoculate 400mL LB with a final concentration of 100μg / mL ampicillin at a ratio of 1% (V / V) In the liquid culture medium, shake culture at 37°C and 200rpm. Waiting for OD 600 When between 0.8-1.0, the inducer IPTG (isopropyl-β-D-thiogalactopyranoside, IPTG) was added at a final concentration of 0.1 mM, and induced overnight at 30°C. The bacteria were collected by centrifugation at 4°C and 8000rpm, then suspended in 50mM pH7.0 sodium phosphate buffer, ultrasonically disrupted (200W, 3s / 5s, 20min), centrifuged at 4°C and 12000rpm for 20min, and the supernatant was taken for freezing After drying, the enzyme freeze-dried powder is obtaine...
Embodiment 3
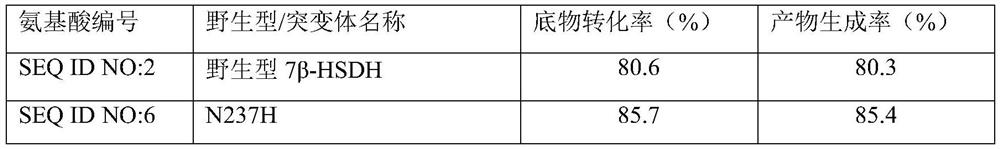
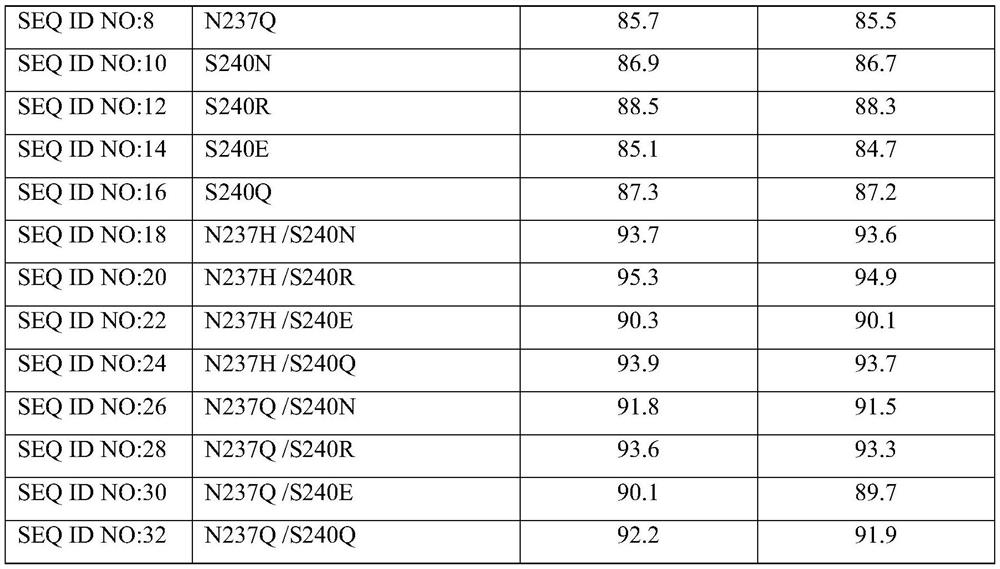
[0068] Construction and screening of embodiment three mutants
[0069] Construction of mutants: The possible beneficial mutation sites were predicted by macromolecular modeling technology as N237 and S240, and site-directed mutations were performed on these two sites (N237K, N237H, N237Q, S240N, S240R, S240E , S240Q). Then use the 7β-HSDH-pET28a(+) recombinant plasmid as a template and use the corresponding synthetic primers to amplify the mutant DNA fragment in the first PCR, and then use the obtained fragment as a template in PCR to amplify the full length of 7β in the second PCR - Mutated gene of HSDH. (For the specific mutation operation, refer to Stratagene’s Site-Directed Mutagenesis Kit Operation Instructions).
[0070] in:
[0071] N237K site mutation (asparagine at position 237 is mutated to lysine)
[0072] Forward primer (SEQ ID NO: 33): 5' TCGCCGGTCAACGTAAAAAAGATAGCGTCC 3',
[0073] Reverse primer (SEQ ID NO: 34): 5'GGACGCTATCTTTTTTACGTTGACCGGCGA 3';
[007...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com