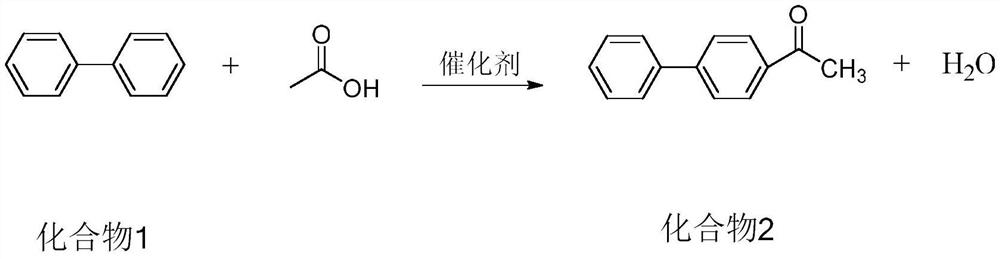
Preparation method and application of p-phenylacetophenone
A technology of phenylacetophenone and biphenyl, which is applied in the field of preparation of p-phenylacetophenone, can solve the problems of not being able to meet market demand, increase production cost expenditure, environmental impact, etc., achieve large specific surface area, reduce Production cost, the effect of reducing industrial production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of embodiment 1 H type molecular sieve catalyst
[0040] Concrete preparation steps are as follows:
[0041] Dissolve 100g of silica sol in 200g of distilled water, dissolve 2.84g of sodium aluminate and 2.32g of strong sodium oxide in 80g of distilled water to form an alkaline sodium aluminate solution. At a temperature of 25-30°C, add the aqueous silica sol solution dropwise to the alkaline sodium aluminate solution, keep stirring for 5.0-6.0h, then add it to the crystallization autoclave, and crystallize at 200°C for 50h. Then filter under reduced pressure, and the refined high-purity crystals are calcined at 400-450° C. for 3.0-4.0 hours to obtain ordinary Na-ZSM-5 zeolite.
[0042] Then, under the condition of 100-120°C, put Na-ZSM-5 zeolite in ammonium nitrate solution (2mol / L) and stir for 5.0h to obtain preliminary H-ZSM-5 zeolite, and add 30% sulfuric acid solution after filtration , stirred at 50°C for 2.0h, filtered, calcined at 250-300°C fo...
Embodiment 2
[0044]The synthesis of embodiment 2 p-phenylacetophenone
[0045] Proceed as follows:
[0046] Add 315g DMSO in 1000ml autoclave, add biphenyl (1.0mol, 157.36g), acetic acid (1.3mol, 79.66g), H-type molecular sieve catalyst 9.44g (6% of biphenyl mass).
[0047] Raise the temperature to 180°C, pressurize to 0.6MPa, stir and react for 5.0h, lower the temperature and pressure, take samples for analysis, and the reaction is complete.
[0048] The dehydration catalyst was removed by filtration, DMSO and acetic acid were removed by vacuum distillation, 315 g of dichloroethane and 50 g of water were added, the mixture was stirred and left to separate layers, and the organic phase was spin-dried to obtain 193.78 g of the product with a purity of 96.50% and a yield of 95.41%.
Embodiment 3
[0050] Proceed as follows:
[0051] Add 315g DMSO in 1000ml autoclave, add biphenyl (1.0mol, 157.36g), acetic acid (1.5mol, 91.92g), H-type molecular sieve catalyst 12.59g (8% of biphenyl mass).
[0052] Raise the temperature to 180°C, pressurize to 0.6MPa, stir and react for 5.0h, lower the temperature and pressure, take samples for analysis, and the reaction is complete.
[0053] The dehydration catalyst was removed by filtration, DMSO and acetic acid were distilled off under reduced pressure, 315 g of dichloroethane and 50 g of water were added, stirred and left to separate, and the organic phase was spin-dried to obtain 193.89 g of the product with a purity of 95.42% and a yield of 94.39%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com