A kind of ultra high performance liquid chromatography analysis method of semaglutide
An ultra-high performance liquid chromatography and chromatographic analysis technology, applied in the field of peptide quality analysis, can solve the problems of unclear toxicological data, affecting the product quality of semaglutide, and the risk of patients' medication, increasing the number of impurities and improving the permeability. , the effect of increasing the number of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
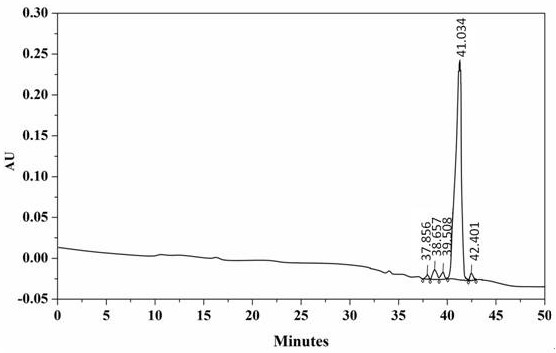
Examples
Embodiment 1
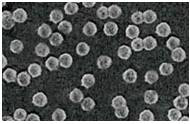
[0053] Preparation of core-shell fillers:
[0054] Preparation and Surface Modification of Non-porous Silica Microspheres
[0055] Mix absolute ethanol, deionized water, and ammonia water at a volume ratio of 15:0.7:1, and ultrasonically disperse for 12 minutes to obtain liquid A; take tetraethyl orthosilicate and add it to absolute ethanol at a volume ratio of 1:8 Make a dilute solution, sonicate for 15 minutes to obtain liquid B; slowly add liquid B to liquid A, the volume ratio of liquid A and liquid B is 2.1:1, add dropwise in 15 hours, then react at room temperature for 4 hours; remove by centrifugation Alkaline solution, deionized water, vacuum filtration and washing until the filtrate is neutral, then washing with ethanol 4 times, and vacuum drying at 65 °C for 8 h to obtain non-porous silica microspheres;
[0056] Disperse non-porous silica microspheres in absolute ethanol at a concentration of 0.02 g / mL, add γ-ureapropyltrimethoxysilane, in which non-porous silica mi...
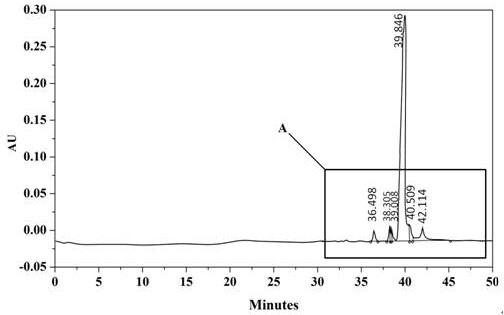
Embodiment 2
[0062] The difference between the preparation of core-shell type filler and embodiment 1 is:
[0063] Core-shell SiO 2 During the preparation of microspheres, the mass ratio of surface-modified non-porous silica microspheres to urea was 1:1.1; the solid-liquid ratio of modified non-porous silica microspheres to silica sol solution was 1 g:1.6 mL ; The solid-liquid ratio of modified non-porous silica microspheres to formaldehyde solution was 1 g: 1.2 mL; the mass ratio of 2-aminopyrimidine-5-formaldehyde hydrate to urea was 1: 1.7.
[0064] Core-shell SiO 2 During the bonding process on the surface of microspheres, core-shell SiO 2 The mass ratio of microspheres and octadecyltrichlorosilane is 1:1.15.
Embodiment 3
[0066] The difference between the preparation of the core-shell filler and Example 1 is that the core-shell SiO 2 During the preparation of the microspheres and the surface bonding process, a new type of organosilane is added, specifically:
[0067] core-shell SiO 2 The microspheres were added to hydrochloric acid solution (hydrochloric acid / water = 1, v / v) with a concentration of 20 g / mL, stirred and activated in an oil bath at 138 °C for 7 h after ultrasonication for 8 min, and then deionized water was filtered and washed until neutral , dried in vacuum at 120 °C for 10 h; then added the dried toluene (core-shell SiO 2 The solid-to-liquid ratio of microspheres to toluene was 1 g: 30 mL), after ultrasonic dispersion for 6 min, octadecyltrichlorosilane (core-shell SiO 2 The mass ratio of microspheres, octadecyltrichlorosilane is 1:1.24) and new organosilanes (with core-shell SiO 2 The mass ratio of the microspheres was 1:0.42), stirred and refluxed for 22 h in an oil bath a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com