Pichia pastoris genetically engineered bacterium for heterologous expression of recombinant catalase and application of pichia pastoris genetically engineered bacterium
A technology of catalase and genetically engineered bacteria, applied in the direction of genetic engineering, oxidoreductase, application, etc., can solve the problems of reduced stability, complicated production process, and reduced target protein output, so as to reduce production and use costs , The separation and purification process is simple, and the effect of promoting large-scale preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Construction of pPICZαA-KatA expression vector
[0041] Synthesize the following primers respectively:
[0042] Primer pPICZαA-KatA-F:
[0043] SEQ ID NO.2:
[0044] 5'-AGGGGTATCTCTCGAGAAAAGAAGTTCAAATAAACTGACAACTAGCTGG-3'
[0045] Primer pPICZαA-KatA-R:
[0046] SEQ ID NO.3:
[0047] 5’-TCAATGATGATGATGATGATGAGAATCTTTTTTAATCGGCAATCCAAGGCCTTCT GCCAC-3’
[0048] Using RF cloning technology, under the guidance of the above primers, the plasmid pMD 19-T-KatA (nucleotide sequence shown in SEQ ID NO.1) preserved in the laboratory was used as a template to carry out the second One round of PCR amplification obtained the KatA gene containing the homology arm of the pPICZαA vector. The obtained PCR products were separated by 1% agarose gel electrophoresis, and purified and recovered by using a DNA gel recovery kit. Then, the long fragment obtained from the first round of PCR reaction was used as a primer, and the plasmid pPICZαA was used as a template to amplify ...
Embodiment 2
[0049] 6. Example 2 Heterologous expression of recombinant catalase
[0050] The recombinant expression plasmid pPICZαA-KatA obtained in Example 1 was linearized with the restriction endonuclease Sac I, and after electrophoresis was verified to be correct, the recovered linearized recombinant plasmid was transformed into Pichia pastoris X by electroporation -33 competent cells were spread on YPD solid plate containing 100 μg / ml zeocin antibiotic and cultured at 30°C for 3-4 days. Pick a single colony with a smooth surface and a good shape from the plate and place it in 10 ml YPD liquid medium containing 30 μg / ml zeocin at 200 rpm at 30°C for 24 hours to obtain a seed solution. Then take 1ml of the above seed solution and put it into 100ml of BMGY medium, culture at 200rpm, 30°C for 16-18h. Centrifuge 5000 g of the bacterial solution obtained from BMGY medium at 4°C for 20 minutes, discard the supernatant, resuspend in 200 mL of BMMY medium, and cultivate at 200 rpm at 30°C. ...
Embodiment 3
[0051] Embodiment 3 Separation and purification of recombinant catalase
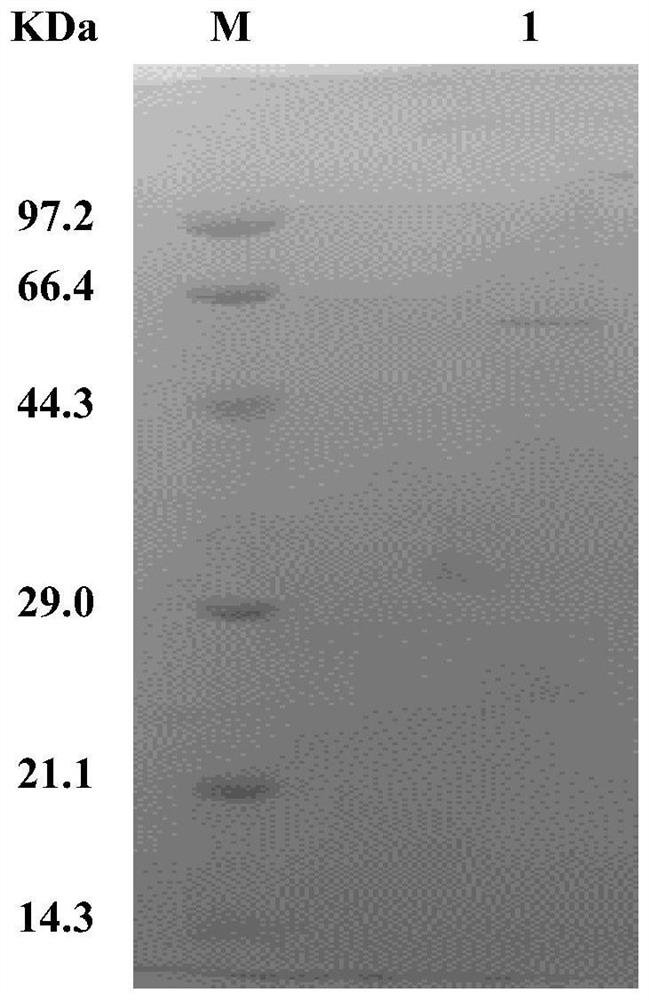
[0052] The fermented bacterial liquid was centrifuged at 10,000 g at 4°C for 20 min, and the extracellular supernatant was collected and concentrated by ultrafiltration and buffer exchange, so that the protein was finally stored in 50 mM phosphate buffer at pH 7.0. Subsequently, the above protein samples were subjected to DEAE anion exchange chromatography, and linear gradient elution was carried out through an elution buffer containing NaCl, and the eluted samples were detected by SDS-PAGE electrophoresis, and the results were as follows figure 1 As shown, lane M is the protein molecular weight standard, and lane 1 is the eluted sample. A single band appears between 44.3-66.4kDa, which is consistent with the expected relative molecular mass of the target protein of 55.5kDa, indicating that the recombinant catalase has been successfully purified .
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com