Composition for loosening bowel to relieve constipation and preparation method thereof
A technology for laxative and laxative composition, applied in the field of laxative and laxative composition and preparation thereof, can solve the problems of large difference in drug filling amount, reduced drug stability, large difference in dosage, etc., and achieves improved quality Controllability, improved material dispersion, improved material properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Take aloe vera and crush it, pick it up to remove impurities, dry it in a drying oven at 110±10°C for 10 hours, crush it, and pass it through a 70-mesh sieve to obtain aloe vera powder, weigh 143g; remove impurities from amber, rinse with drinking water, and pour clean water Separate, dry in a drying oven at 110±10°C for 10 hours, pulverize, pass through a 70-mesh sieve to obtain amber medicinal powder, weigh 143g; crush Qingdai, pass through a 70-mesh sieve and 200-mesh sieve to control the particle size, put on a tray and dry under the same drying conditions Before, to obtain Qingdai powder, weigh 143g; then weigh 8.75g of croscarmellose sodium, put it in a CH-600 three-dimensional blender and mix evenly, then weigh 2.25g of magnesium stearate and add to mix evenly. Filling, polishing, aluminum plastic, and laxative hard capsules.
Embodiment 2
[0032] Acute toxicity test:
[0033] Get 20 mice, half male and half male, free to drink water, after fasting for 12 hours, gavage 10 g / kg of the capsule powder obtained in Example 1, with an interval of 8 hours, twice in total, and the daily cumulative amount is 20 g / kg. After administration, the animals were observed for signs of acute poisoning and death, and then the animals were routinely fed for one week to observe their general state and record their body weight. Results No adverse reactions occurred after administration, and no animal died. The mice were fed routinely for one week, and the general condition was good, and the food intake, drinking water, defecation, and urination were all normal. The average body weight increased from 20.8±0.7g to 25.6±1.7g. At the end of the experiment, all the animals were sacrificed, and the main organs were observed with naked eyes after dissection, and there was no abnormal change visible to the naked eye.
Embodiment 3
[0035] Functional ingredient content detection
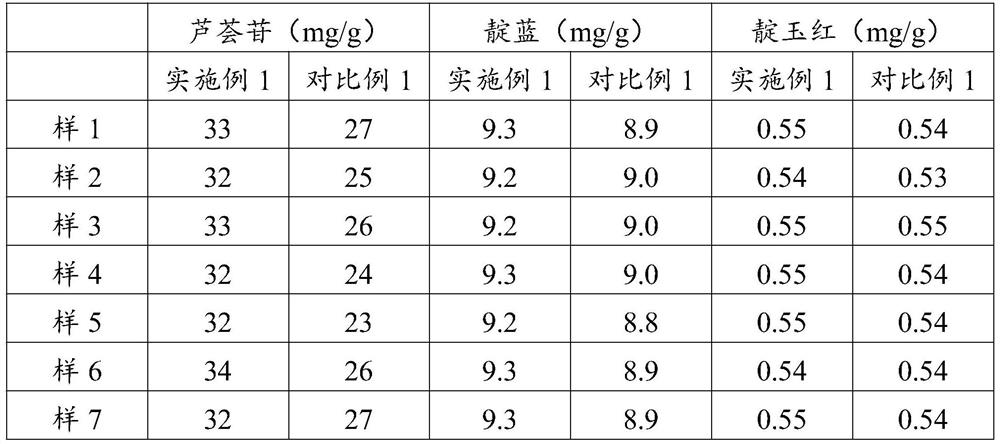
[0036] The contents of the active ingredients aloin, indigo and indirubin contained in the Runchang Tongbian capsules obtained in Example 1 and Comparative Example 1 were detected by high performance liquid chromatography.
[0037] 1. Detection method of aloin:
[0038] Preparation of the test solution--the content under the difference in loading amount, finely grind, take about 0.1g, weigh it accurately, put it in a 50ml measuring bottle, add an appropriate amount of methanol, sonicate for 30 minutes, let it cool, add methanol to the mark , Shake well, filter, take the continued filtrate, that is.
[0039]Preparation of the reference substance solution-accurately weigh an appropriate amount of the aloin reference substance, put it in a brown measuring bottle, add methanol to make a solution containing 0.1mg per 1ml, and obtain it.
[0040] Determination:
[0041] Precisely draw 10 μl of each of the above two solutions, injec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com