Proton pump inhibitor capsule and preparation method thereof
A technology of proton pump inhibitors and capsules, which is applied in the field of proton pump inhibitor capsules and its preparation, can solve the problems of complex production process of proton pump inhibitors, unstable drug release process, large adverse reactions, etc., and is suitable for mass production Preparation, inhibition of hypersecretion of gastric acid, and effect of improving gastrointestinal diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
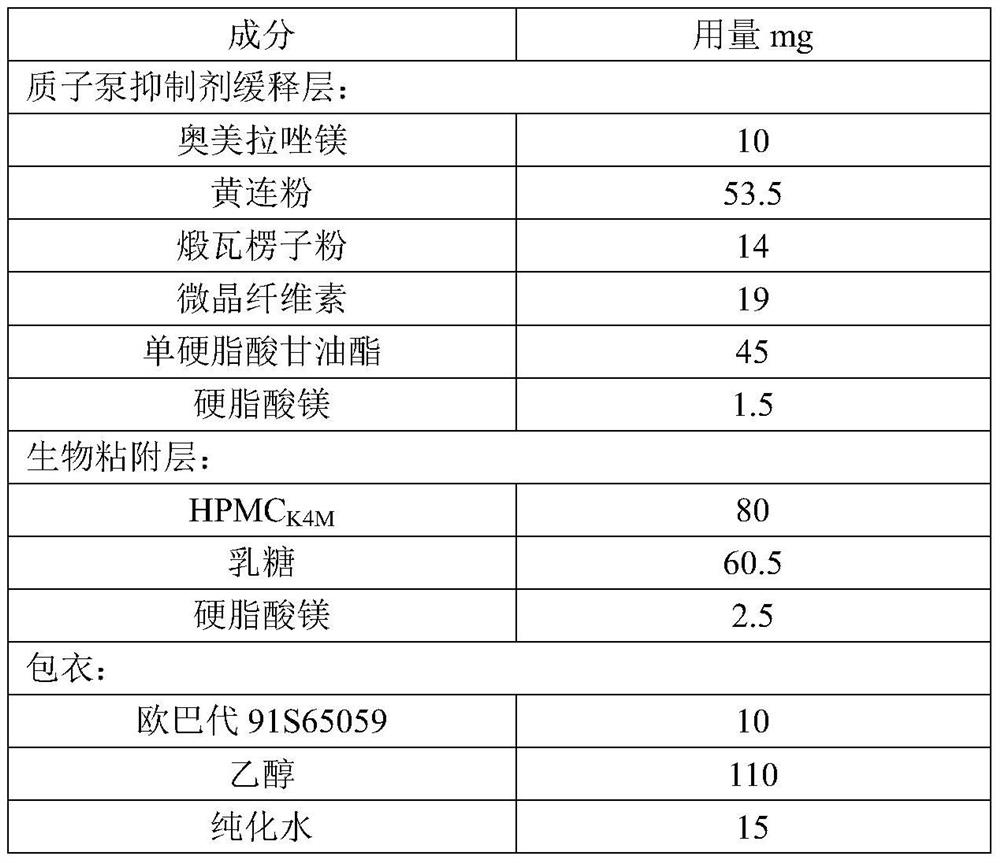
[0045] This embodiment provides a proton pump inhibitor capsule, including a capsule shell and enteric-coated microtablets, and the materials of the enteric-coated microtablets are shown in Table 1 below:
[0046] Table 1 Example 1 enteric-coated microtablet ingredients and dosage
[0047]
[0048] The specific preparation method is as follows:
[0049] (1) Omeprazole magnesium is premixed with Coptidis rhizome powder and calcined corrugated seed powder, then microcrystalline cellulose and glyceryl monostearate are mixed, and finally magnesium stearate is mixed for subsequent use;
[0050] (2) HPMC K4M and lactose are put into wet granulation, and after drying, the water content is controlled within 5%, and then magnesium stearate is put into the granules for final mixing for later use;
[0051] (3) Take steps (1) and (2) to obtain the pre-product, and use a rotary tablet press to compress the tablet to obtain the micro-tablet body, with a diameter of 0.5-5mm, a tablet th...
Embodiment 2
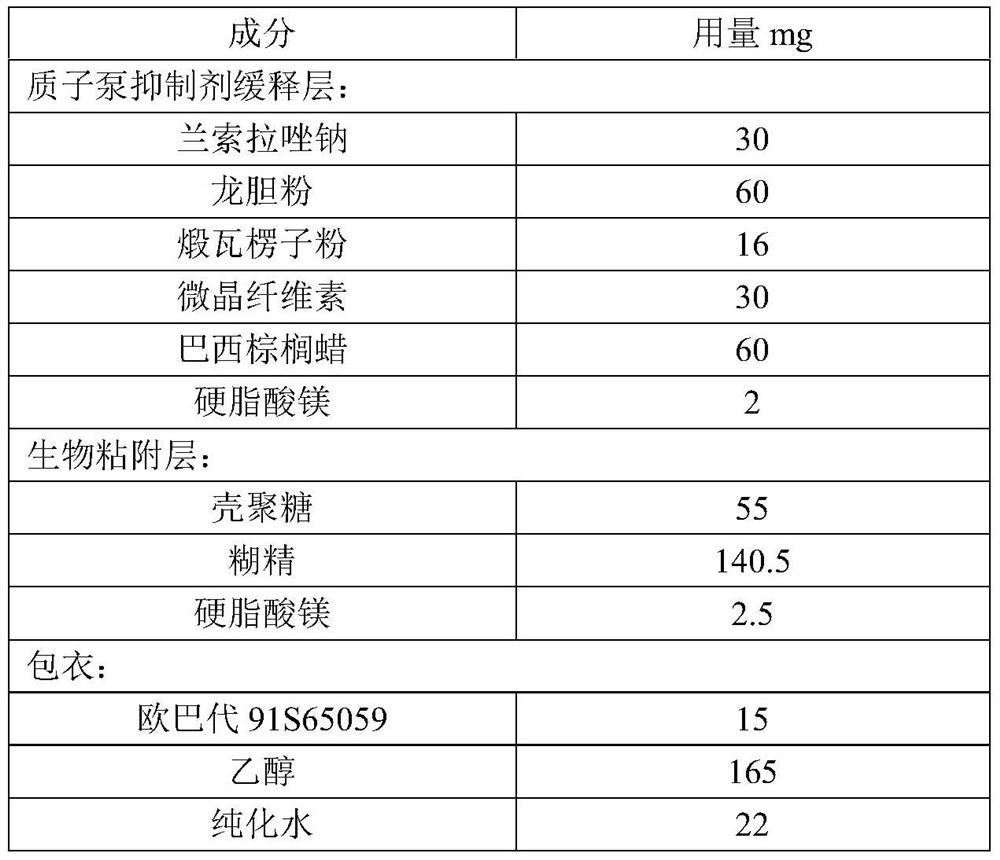
[0055]This embodiment provides a proton pump inhibitor capsule, including a capsule shell and enteric-coated microtablets, and the materials of the enteric-coated microtablets are shown in Table 2 below:
[0056] Table 2 Example 2 enteric-coated microtablet ingredients and dosage
[0057]
[0058] The specific preparation method is as follows:
[0059] (1) Lansoprazole sodium is premixed with gentian powder and calcined corrugated seed powder, then put into microcrystalline cellulose and carnauba wax and mix, finally drop into magnesium stearate and mix for subsequent use;
[0060] (2) Putting chitosan and dextrin into wet granulation, controlling the moisture within 5% after drying, and then putting magnesium stearate into the granules for final mixing for subsequent use;
[0061] (3) Take steps (1) and (2) to obtain the pre-product, and use a rotary tablet press to compress the tablet to obtain the micro-tablet body, with a diameter of 0.5-5mm, a tablet thickness of 1-5m...
Embodiment 3
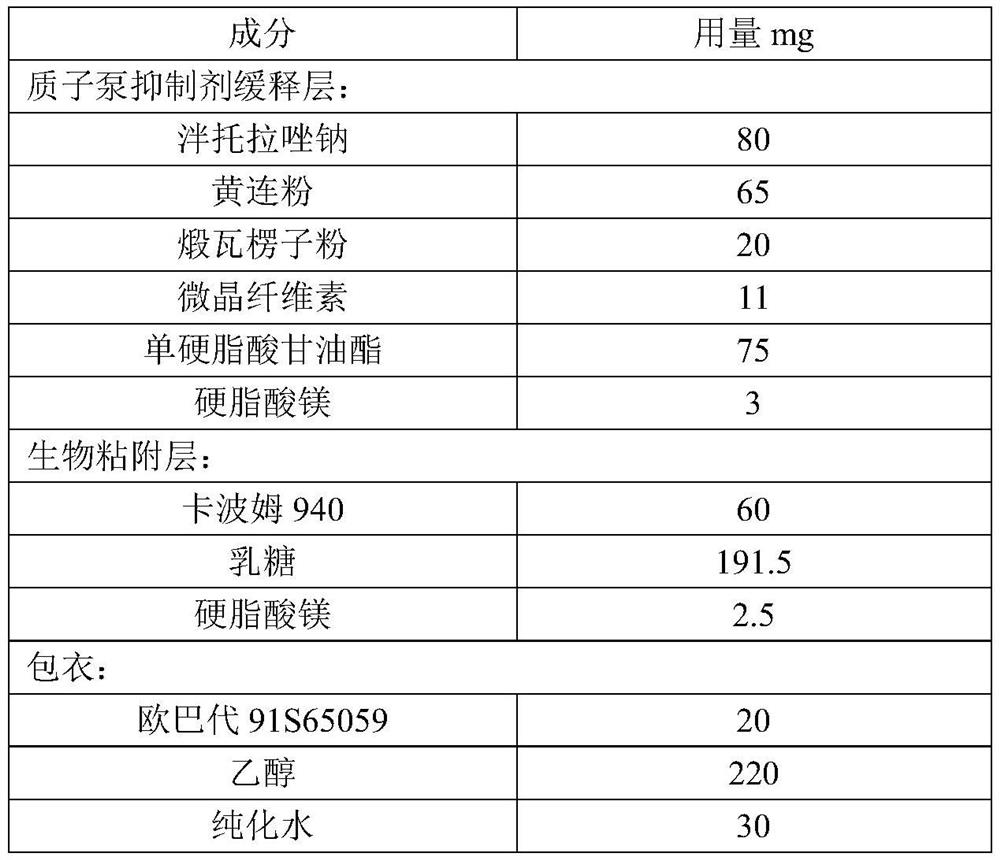
[0065] This embodiment provides a proton pump inhibitor capsule, including a capsule shell and enteric-coated microtablets, and the materials of the enteric-coated microtablets are shown in Table 3 below:
[0066] Table 3 Example 3 enteric-coated microtablet ingredients and dosage
[0067]
[0068] The specific preparation method is as follows:
[0069] (1) Pantoprazole Sodium is premixed with Coptidis Rhizoma Coptidis powder, calcined corrugated seed powder, then drop into microcrystalline cellulose and glyceryl monostearate and mix, finally drop into magnesium stearate and mix for subsequent use;
[0070] (2) Carbomer 940 and lactose are put into wet granulation, and after drying, the water content is controlled within 5%, and then magnesium stearate is put into the granules for final mixing for later use;
[0071] (3) Take steps (1) and (2) to obtain the pre-product, and use a rotary tablet press to compress the tablet to obtain the micro-tablet body, with a diameter of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com