Tyrosine phenol lyase mutant, engineering bacterium and application of tyrosine phenol lyase mutant in catalytic synthesis of levodobar
A tyrosine phenol, levodopa technology, applied in the directions of lyase, carbon-carbon lyase, application, etc., can solve the problems of enzyme inactivation, enzyme and cytotoxicity, etc., and achieve the effect of excellent catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1, the acquisition of TPL gene
[0026] Extract the whole genome DNA of Fusobacterium nucleatum (F. nucleatum subsp.CGMCC 1.2526, purchased from China Industrial Microorganism Culture Collection Management Center) with a DNA extraction kit (purchased from Thermo Fisher Scientific Company), and use the DNA as a template, upstream The primer (5'TGTTAGCAGCCGGATCTCAGT3') and the downstream primer (5'GGAGATATACCATGCGCTTTGA3') were used as primers for PCR amplification. Addition amount of each component of the PCR reaction system (total volume 50 μL): 5×PrimeSTARTM HS DNA polymerase Buffer 10 μL, 10 mM dNTP mixture (dATP, dCTP, dGTP and dTTP each 2.5 mM) 4 μL, 50 μM upstream primer and downstream primer 1 μL each , genomic DNA 1 μL, PrimeSTARTM HS DNA polymerase 0.5 μL, nucleic acid-free water 32.5 μL. The PCR reaction conditions were as follows: pre-denaturation at 95°C for 5 minutes, followed by a temperature cycle of 95°C for 1 minute, 55°C for 1 minute, and 72...
Embodiment 2
[0027] Embodiment 2, error-prone PCR construction TPL mutant library
[0028] Using the TPL gene obtained in Example 1 as a template, the mutant sequence was obtained by error-prone PCR amplification. The amplification primers are:
[0029] (5'TGTTAGCAGCCGGATCTCAGT3') and (5'GGAGATATACCATGCGCTTTGA3').
[0030] The amplification system is: 50μl reaction system:
[0031] 10×Taq polymerase buffer: 5 μL; Mg 2+ (25mM): 2-16μL; Mn 2+ (5mM): 2-20μL; 10mM dNTP mixture (dATP, dCTP, dGTP and dTTP each 2.5mM) 4μl; concentration of 50μM upstream primer, downstream primer 1μL each, DNA template: 1μL; Taq DNA polymerase: 0.5μL; Make up the system with double distilled water.
[0032] The PCR reaction conditions were as follows: pre-denaturation at 95°C for 5 minutes, followed by a temperature cycle of 95°C for 1 minute, 55°C for 1 minute, and 72°C for 90 seconds, a total of 30 cycles, and finally an extension at 72°C for 10 minutes, with a termination temperature of 4°C. The PCR produ...
Embodiment 3
[0033] Embodiment 3, the screening of TPL mutant library
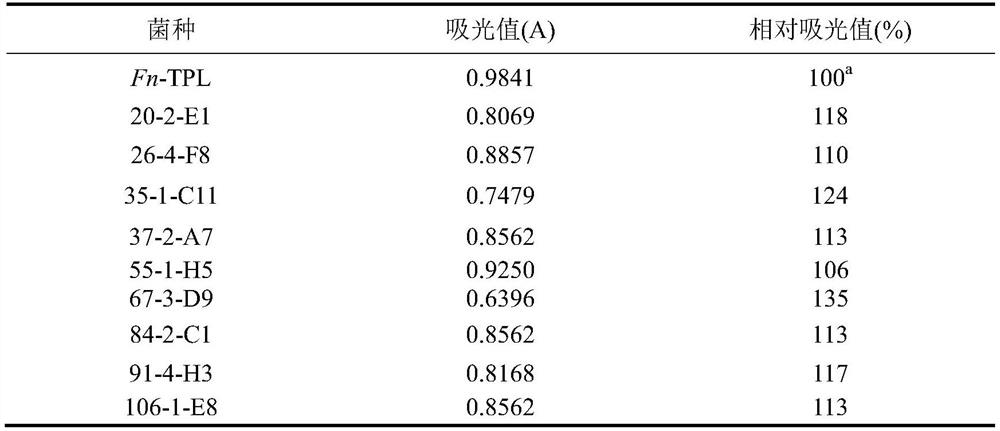
[0034] The TPL mutation library constructed in Example 2 was screened by salicylaldehyde spectrophotometry. The principle of color development is: under alkaline conditions, sodium pyruvate and salicylaldehyde will undergo Claisen-Schmidt (Claisen-Schmidt) reaction to generate a yellow compound, the color of which is directly proportional to the content of sodium pyruvate, and then measured by using a spectrophotometer to measure the absorbance of the reaction solution at a specific wavelength.
[0035] The specific reaction steps of color reaction are: in 1mL standard reaction system, add 100μL 250g / L NaOH aqueous solution, 40μL supernatant, 560μL ultrapure water, 20μL salicylaldehyde chromogenic solution in sequence, shake well and add 200μL 250g / L NaOH aqueous solution, 80 μL ultrapure water, after standing at room temperature for 2 hours, take 200 μL of the color reaction solution in a 96-well standard plate, measu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com