Dynamic covalent bond functionalized silver nanowire and preparation method and application thereof
A technology of dynamic covalent bonds and nanowires, which is applied in chemical instruments and methods, nanotechnology, solutions from liquid solvents at room temperature, etc., can solve problems such as easy breakage of silver nanowires and damage to the properties of silver nanowires, and achieve reaction Conditions are easy to control, the length distribution is uniform, and the operation is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
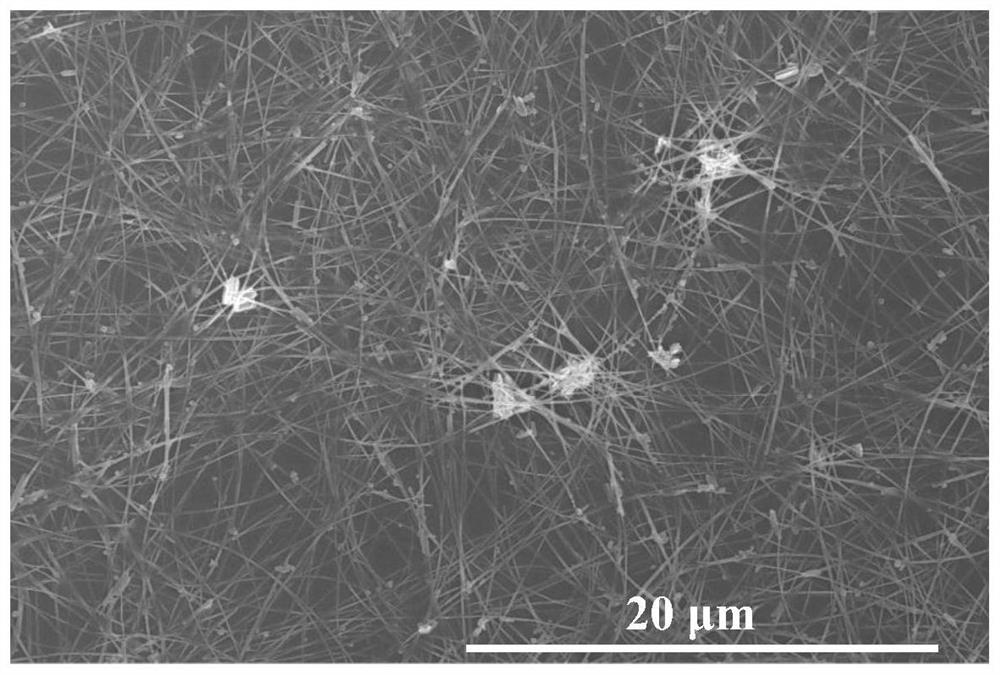
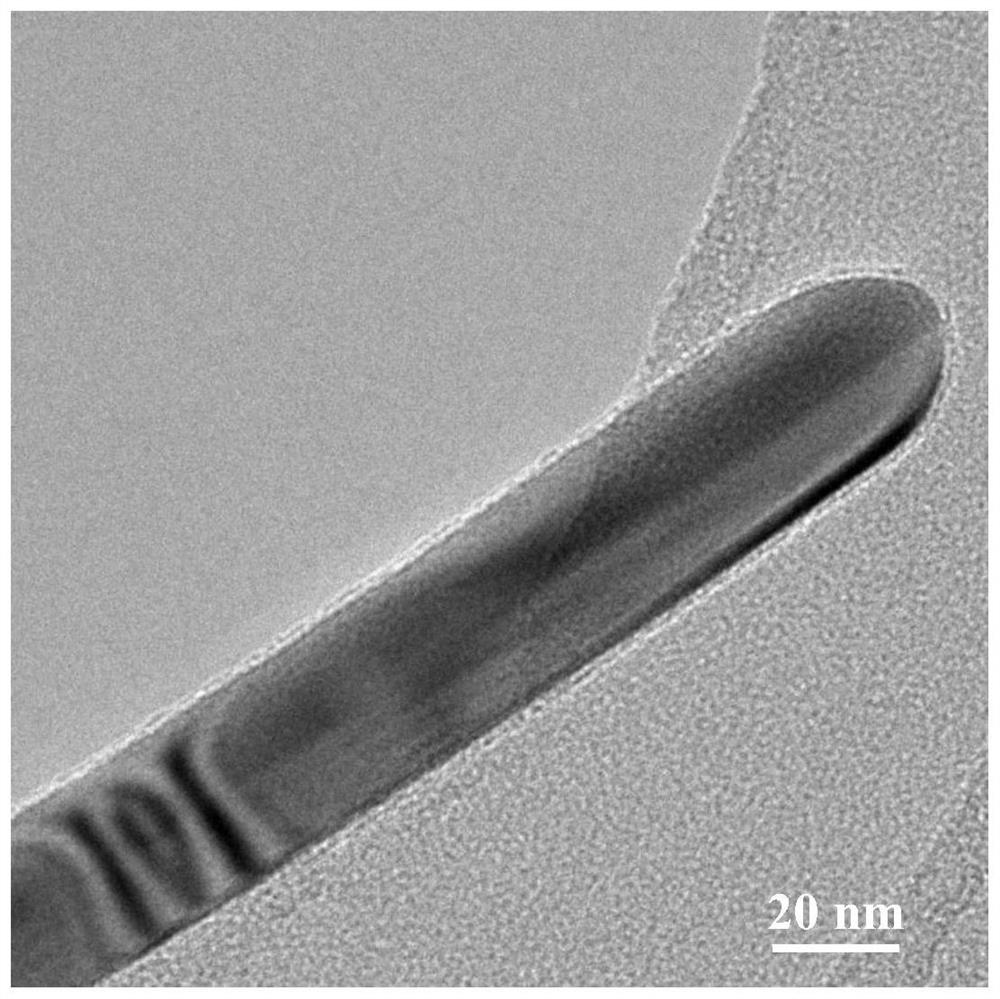
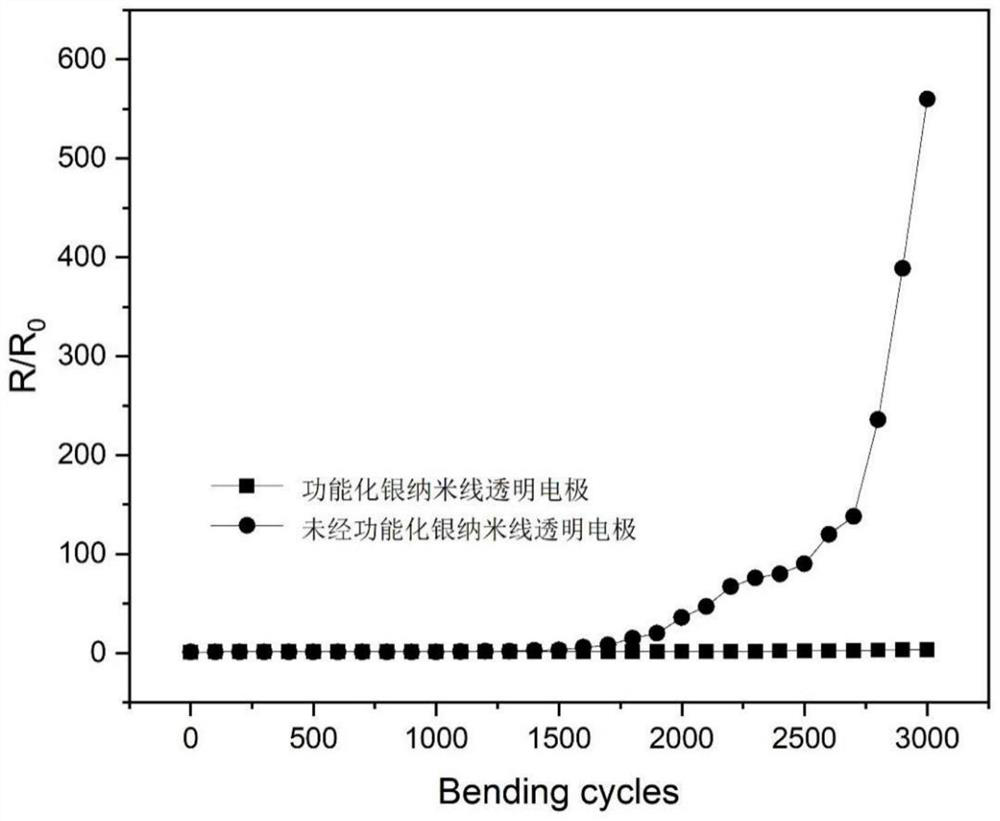
Image
Examples
Embodiment 1
[0052] (17.78 g, 160 mmol) N-vinylpyrrolidone, (9.8 g, 40 mmol) 2,3-dioxane methyl methacrylate, (12.3 mg, 0.075 mmol) azo diisobutyronitrile (AIBN) is dissolved in 15 ml of benzyl ether. The air was replaced with nitrogen bubbles, and the solution was heated for 30 minutes to 65 ° C, and the reaction was 16 hours. 10 ml of tetrahydrofuran (THF) was added to the reaction system, and then dried diethyl ether precipitates the product to obtain polymer A.
[0053]
[0054] Then, 2 g of a NaBR (solvent having 1 ml 2 mmol / L), 1 ml 2 mmol / L of NaCl (solvent is ethylene glycol), respectively, is added to a solution containing 100 ml of concentration of 0.025 g / ml polymer A. (The solvent is ethylene glycol) to give a solution A; 1 g of silver nitrate is continued to the solution A, and the bubble is bubbled for 10 minutes, and the reactor is sealed, and the oven is added to 170 ° C, and the holding is 5 hours. The reaction was stopped, cooled to room temperature, add 200 ml of eth...
Embodiment 2
[0056] (17.78 g, 160 mmol) N-vinylpyrrolidone, (11.5 g, 40 mmol) 5,6-dioxane methyl methacrylate, (12.3 mg, 0.075 mmol) azo diisobutyronitrile was dissolved in 15 ml In the ether. The air was replaced with nitrogen bubbles, and the solution was heated for 30 minutes to 65 ° C, and the reaction was 16 hours. 10 ml of tetrahydrofuran was added to the reaction system, and then dried diethyl ether precipitates the product to obtain polymer A.
[0057] Then, 2 g of a NaBR (solvent having 1 ml 2 mmol / L), 1 ml 2 mmol / L of NaCl (solvent is ethylene glycol), respectively, is added to a solution containing 100 ml of concentration of 0.025 g / ml polymer A. (The solvent is ethylene glycol) to give a solution A; 1 g of silver nitrate is continued to the solution A, and the bubble is bubbled for 10 minutes, and the reactor is sealed, and the oven is added to 170 ° C, and the holding is 5 hours. The reaction was stopped, cooled to room temperature, add 200 ml of ethanol centrifugation to ob...
Embodiment 3
[0059] (17.78 g, 160 mmol) N-vinylpyrrolidone, (19.6 g, 80 mmol) 2,3-dioxane methyl methacrylate, (12.3 mg, 0.075 mmol) azo diisobutyronitrile was dissolved in 15 mL benzoanitrile In the ether. The air was replaced with nitrogen bubbles, and the solution was heated for 30 minutes to 65 ° C, and the reaction was 16 hours. 10 ml of tetrahydrofuran was added to the reaction system, and then dried diethyl ether precipitates the product to obtain polymer A.
[0060] Then, 2 g of a NaBR (solvent having 1 ml 2 mmol / L), 1 ml 2 mmol / L of NaCl (solvent is ethylene glycol), respectively, is added to a solution containing 100 ml of concentration of 0.025 g / ml polymer A. (The solvent is ethylene glycol) to give a solution A; 1 g of silver nitrate is continued to the solution A, and the bubble is bubbled for 10 minutes, and the reactor is sealed, and the oven is added to 170 ° C, and the holding is 5 hours. The reaction was stopped, cooled to room temperature, add 200 ml of ethanol centri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com