Nitrogen oxide light-emitting particles, preparation method and light-emitting device
A nitrogen oxide, luminescent particle technology, applied in chemical instruments and methods, luminescent materials, semiconductor devices, etc., can solve the problems of decreased luminous intensity of phosphors, narrow excitation range, low luminous efficiency of phosphors, etc., and achieve a solid crystal structure. and stability, the manufacturing method is simple and easy, and the anti-aging and light decay performance is good.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0069] The preparation method 1 of a nitrogen oxide luminescent particle and its preferred solution proposed by the present invention includes the following specific steps:
[0070] Step 1: Use the nitrides, oxides or halides of M, A, B, R as raw materials, according to the general chemical formula M m-m1 A a1 B b1 o O1 N n1 :R m1 The stoichiometric ratio of the cations in the composition weighs the required raw materials;
[0071] Step 2: Mix the raw materials weighed in step 1 uniformly in a nitrogen atmosphere to form a mixed material; wherein the mixing time of the raw materials is 1-5 hours;
[0072] Step 3: Roast the mixture obtained in step 2 at a high temperature in a roasting atmosphere, then lower the temperature to a predetermined temperature, and then pass in nitrogen-oxygen mixed gas or air for low-temperature roasting to obtain a semi-finished product of nitrogen oxide luminescent particles; wherein:
[0073] The calcination temperature is 1400-2000°C, and ...
Embodiment 1
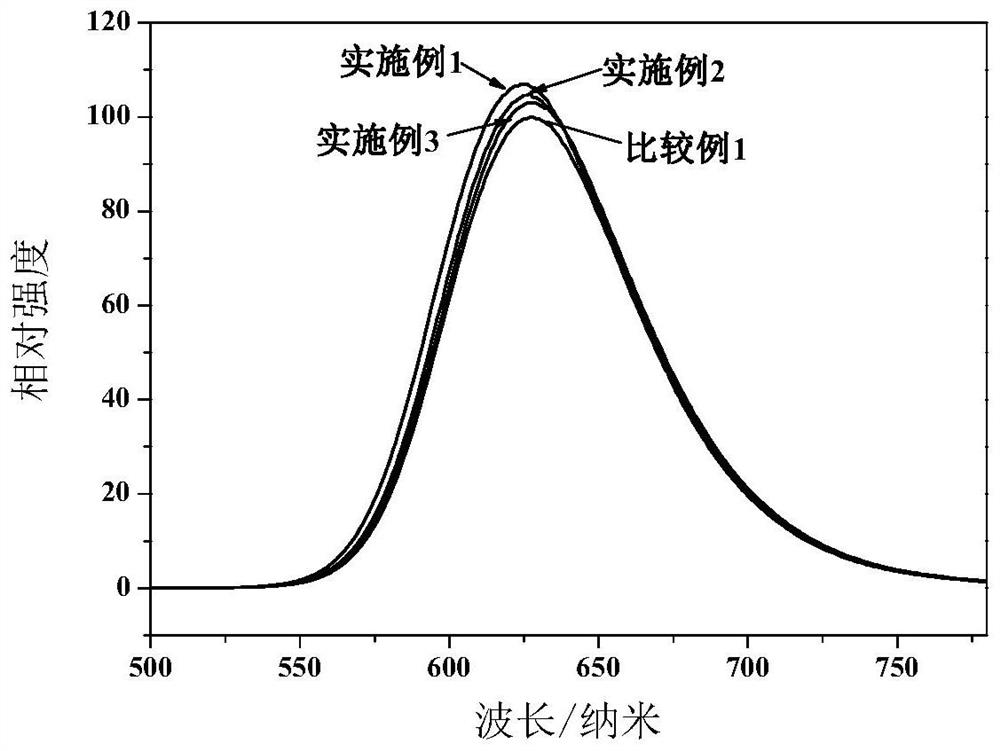
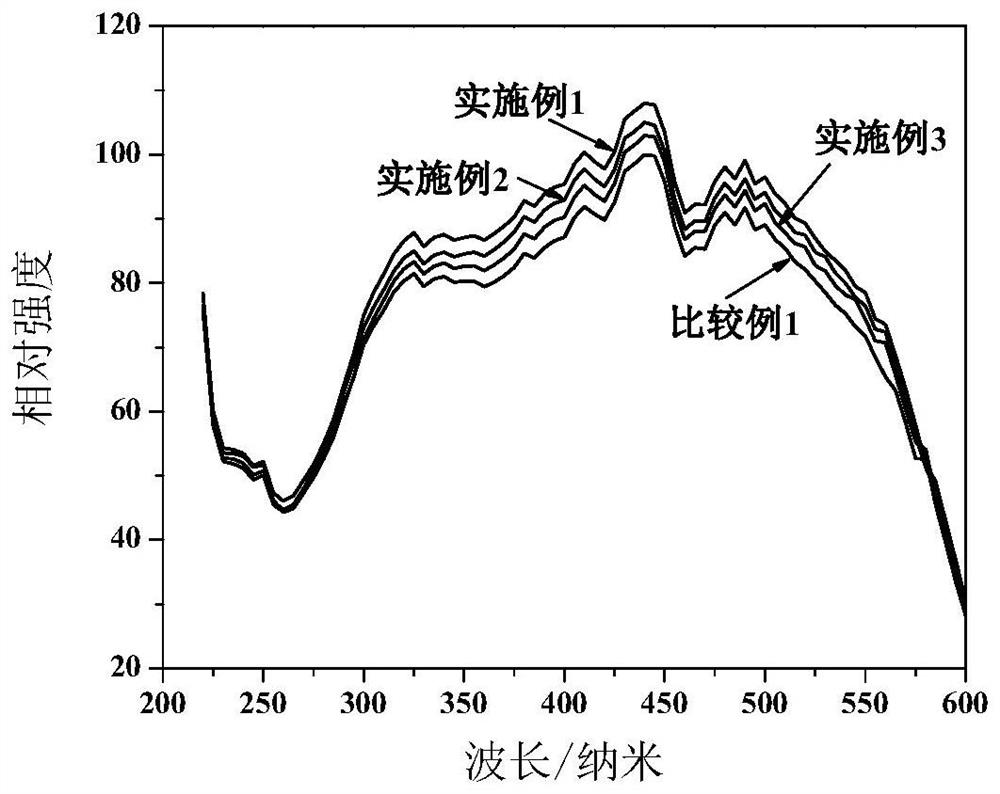
[0087] Weigh Ca 3 N 2 0.319g, Sr 3 N 2 9.288g, AlN4.412g, Si 3 N 4 5.033g, Eu 2 o 3 0.947g, the above raw materials were fully mixed in a nitrogen atmosphere for 2 hours, put into a molybdenum crucible, and then quickly moved into a tube furnace, and then gradually heated up to 1800°C under the protection of a nitrogen atmosphere, and kept for 10h; cooled to 400°C, Pass nitrogen-oxygen mixture gas (20% oxygen by volume) at a speed of 2L / min for roasting, the roasting time is 4h, crush the obtained luminescent particles and sieve, put the sieved luminescent particles into deionized water Stir for 30 minutes, then suction filter, and finally wash until the conductivity is 7.21 μs / cm, and then dry to obtain the finished nitrogen oxide luminescent particles. For the emission spectrum see figure 2 , the excitation spectrum is shown in image 3 , its luminous intensity is shown in Table 1, which is higher than that of Comparative Example 1, and its thermal quenching spectr...
Embodiment 2
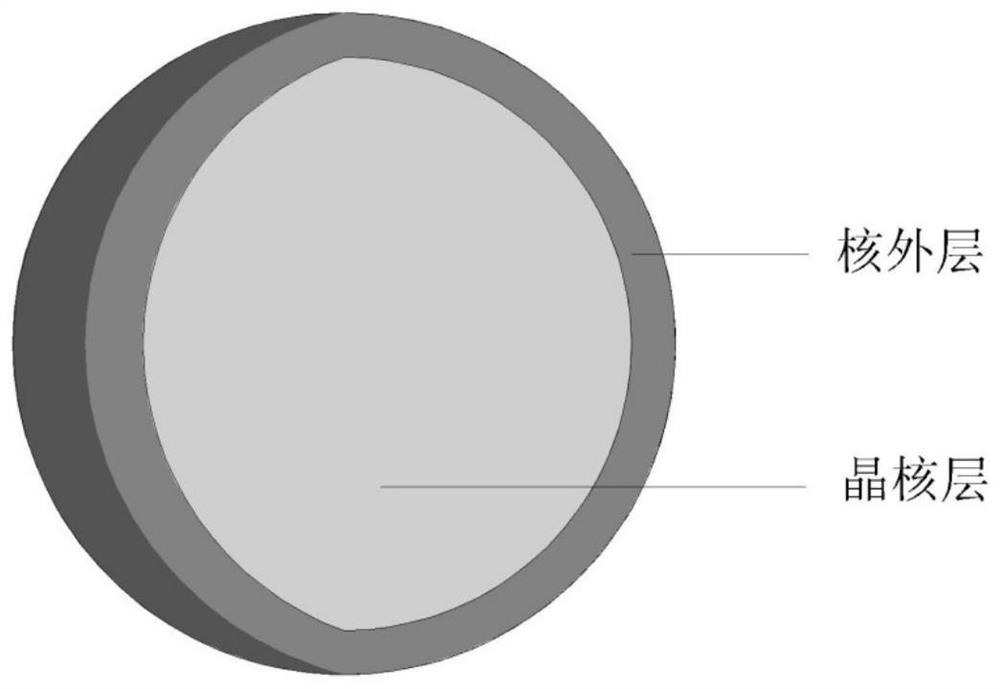
[0089] Weigh Ca 3 N 2 0.537g, Sr 3 N 2 8.963g, AlN4.457g, Si 3 N 4 5.085g, Eu 2 o 3 0.957g, the above raw materials were fully mixed in a nitrogen atmosphere for 2 hours, put into a molybdenum crucible, and then quickly moved into a tube furnace, then gradually heated up to 1800°C under the protection of a nitrogen atmosphere, and kept for 10h; cooled to 400°C, Pass nitrogen-oxygen mixture gas (15% oxygen by volume) at a speed of 2L / min for roasting, the roasting time is 4h, crush the obtained luminescent particles and sieve, put the sieved luminescent particles into deionized water Stir for 30 minutes, then suction filter, and finally wash until the conductivity is 6.12 μs / cm, and dry to obtain the finished nitrogen oxide luminescent particles. For the emission spectrum see figure 2 , the excitation spectrum is shown in image 3 , the thermal quenching spectrum see Figure 4 . The crystal nucleus layer of nitride luminescent particles is Ca 0.1 Sr 0.85 AlSiN 3 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com