Method for preparing beta-nicotinamide mononucleotide through immobilized whole-cell catalysis by taking modified diatomite as carrier
An immobilized cell and single nucleotide technology is applied in the field of immobilized whole cells to catalyze the preparation of β-nicotinamide mononucleotides, which can solve the problems of poor mechanical strength of immobilized whole cells, fewer cycles of use, and high cost of adsorption carriers. problems, to achieve the effects of being not easily degraded by microorganisms, increasing the number of cycles, and not easily falling off cell adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Preparation of fermentation broth
[0045] (1) Strain activation: In a sterile environment, the production strains (Pseudomonas stutzeri) of polyphosphokinase, ribulose-5-phosphate isomerase, phosphoribosyl pyrophosphate synthase and nicotinamide phosphoribosyltransferase bacteria, Penicillium chrysogenum, Bacillus amyloliquefaciens and Pichia pastoris) were added to the plate medium containing 15-25mg / L kanamycin (recipe: tryptone 10g / L, yeast extract 5g) according to the gradient dilution method. / L, sodium chloride 10g / L, agar 12g / L, kanamycin 15-25mg / L, pH=7.0), cultured at 35°C for 20h, and activated.
[0046] (2) Seed liquid preparation: pick any single colony from the cultured plate, and then insert it into the medium containing the 3% inoculum (recipe: tryptone 10g / L, yeast extract 5g / L) , sodium chloride 10g / L, agar 12g / L, pH=7.0) in a shaker flask, put it into a shaker, and cultivate for 15h at 35°C and 180r / min. Then, 3% of the inoculum is insert...
Embodiment 2
[0048] Example 2 Diatomite modification treatment
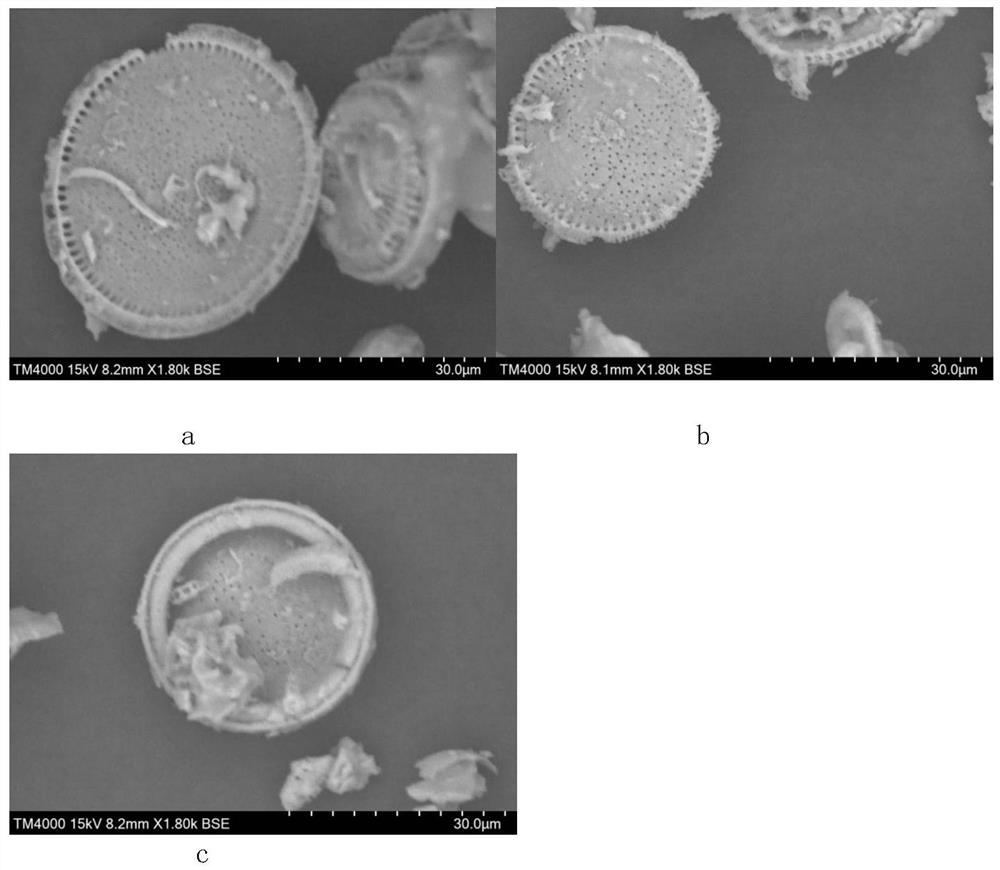
[0049] Acid treatment: prepare 15% hydrochloric acid solution and diatomite (the diatomite used is commercially available FS-500 industrial grade, see the scanning electron microscope picture of the surface structure of diatomite particles figure 1 c) Mix it according to the ratio of 5mL:1g, stir well and soak for 3h. Then filter, rinse the filter cake with ultrapure water until the filtrate is neutral, dry at 110°C and pass through an 80-mesh sieve for use. The SEM image of the surface structure of the obtained diatomite particles is shown in figure 1 the a.
[0050] Alkali treatment: configure 10% sodium hydroxide solution, mix it with the filtrate after drying according to the ratio of 12mL:4g, and soak for 3h. Then filter, rinse the filter cake with ultrapure water until the filtrate is neutral, dry at 110° C. and pass through a 100-mesh sieve to obtain modified diatomite. The SEM image of the surface structure of the...
Embodiment 3
[0056] Example 3 Preparation of immobilized cells
[0057] The bacterial strain fermentation broth obtained in Example 1 was centrifuged for 5 min under the condition of 5000 r / min, respectively, and the lower bacterial sludge was collected, washed with an appropriate amount of deionized water, and centrifuged for 6 min under the same conditions to remove residual bacteria in the bacterial sludge. Fermentation broth and other impurities. After centrifugation, the wet bacterial mud and 0.6% NaCl solution were mixed uniformly in proportion to prepare bacterial suspensions with a concentration of 25% (v / v). Add polyethyleneimine (PEI) solution and stir for 30-50min, then add glutaraldehyde solution, stir and crosslink for 30min. Finally, according to the dry matter content of bacteria mud and the mass ratio of diatomite, it is 1g: 0.5g The modified diatomite obtained in Example 2 was added to carry out adsorption treatment, and after standing for 1-2h, 8-15mM / L MgCl was used 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com