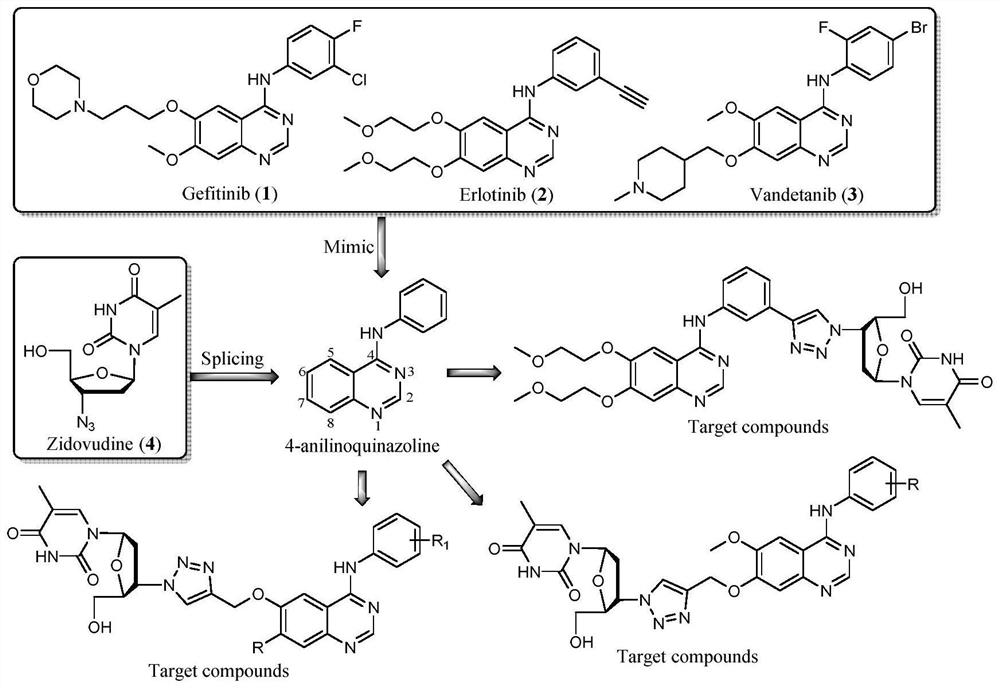
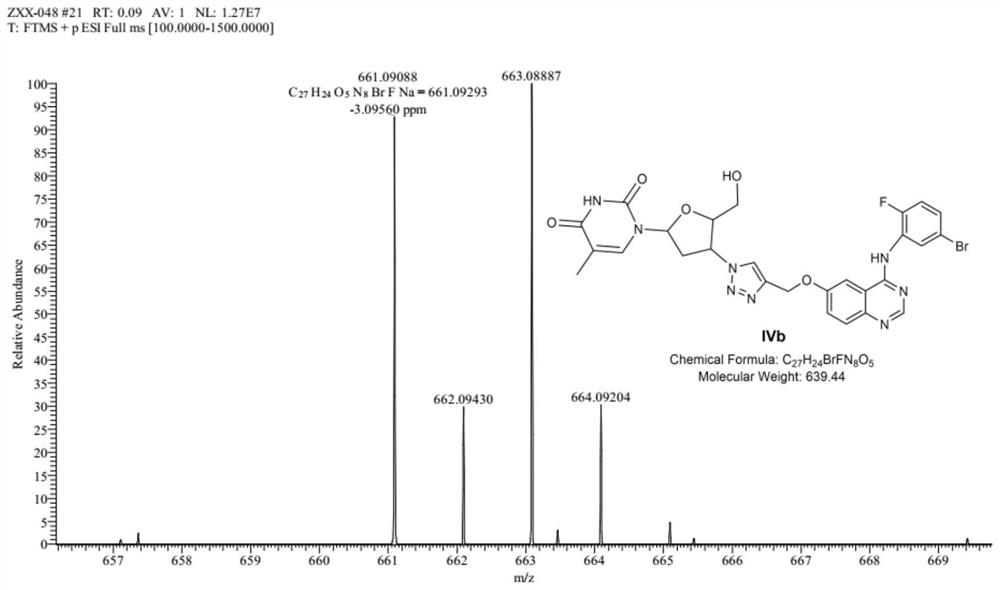
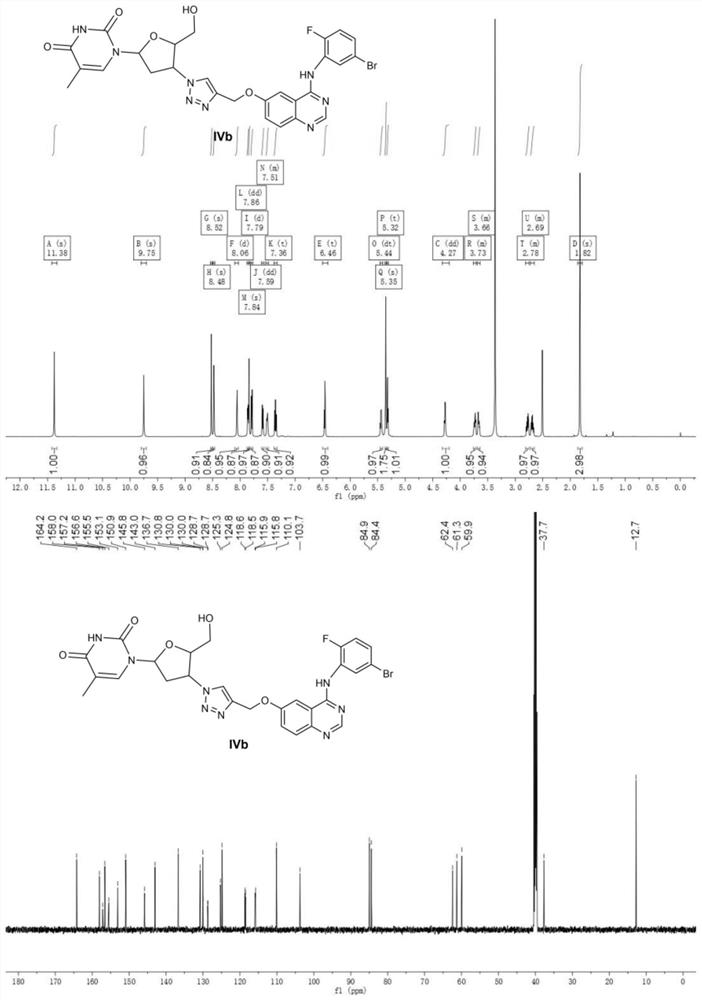
Zidovudine spliced 4-aniline quinazoline compound as well as preparation method and application thereof
A technology of quinazoline and zidovudine, applied in the field of chemistry, can solve the problems of drug resistance, limited selection, and inability to effectively kill cancer cells.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The chemical synthesis of zidovudine splicing 4-aniline quinazoline compounds with general formula (I), the preparation methods are shown in Table 1, the preparation methods of compounds IVa~IVd are exactly the same, but it should be emphasized that the compounds of the present invention are not limited to Contents shown in Table 1.
[0035] Table 1 has a chemical synthesis of formula (I) zidovudine splicing 4-aniline quinazoline compounds
[0036]
[0037] Take the quinazoline intermediate (Ia, 0.10g, 0.32mmol) in a 25mL reaction tube, add 10mL of N,N-dimethylformamide, stir to dissolve it completely, then add anhydrous potassium carbonate (0.13g , 0.95mmol, 3.0eq) and stirred for 10min, slowly added propargyl bromide (45.0mg, 0.38mmol, 1.2eq.), stirred at room temperature, monitored by thin-layer chromatography (TLC), the reaction was complete in about 15 hours, and the reaction solution was added Dilute with 50mL of ethyl acetate, then add 50mL of water for extra...
Embodiment 2
[0043] The chemical synthesis of zidovudine splicing 4-aniline quinazoline compounds with general formula (II), the preparation method is shown in Table 2, the preparation methods of compounds VIIa~VIIb are exactly the same, but it should be emphasized that the compounds of the present invention are not limited to Table 2 shows the content.
[0044] Table 2 has a chemical synthesis of formula (II) zidovudine splicing 4-aniline quinazoline compounds
[0045]
[0046] Take the quinazoline intermediate (Va, 0.10g, 0.31mmol) into a 25mL reaction tube, add 10mL of N,N-dimethylformamide, stir to dissolve it completely, then add anhydrous potassium carbonate (0.13g , 0.95mmol, 3.0eq) and stirred for 10min, slowly added propargyl bromide (45.0mg, 0.38mmol, 1.2eq.), stirred at room temperature, monitored by thin-layer chromatography (TLC), the reaction was complete in about 15 hours, and the reaction solution was added Dilute with 50mL of ethyl acetate, then add 50mL of water for e...
Embodiment 3
[0050] MTT method was used to test the effect of compounds IVa~IVd, VIIa~VIIb on human non-small cell lung cancer cell line (A549), human breast cancer cell line (MCF-7), human cervical cancer cell line (Hela), human liver cancer cell line (HepG2 ), the in vitro anti-tumor activity of human lung adenocarcinoma cisplatin-resistant strain (A549 / DDP), and the description of the test method with A549 cells. But it should be emphasized that the compound of the present invention is not limited to human non-small cell lung cancer cell line (A549), human breast cancer cell line (MCF-7), human cervical cancer cell line (Hela), human liver cancer cell line (HepG2), Cytotoxicity expressed by a cisplatin-resistant strain of human lung adenocarcinoma (A549 / DDP).
[0051] (a) Cell recovery: Take out the A549 cells from the liquid nitrogen, quickly put them into a 37°C water bath, shake the cryopreservation tube gently to dissolve the cryopreservation solution; after dissolving, transfer the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com