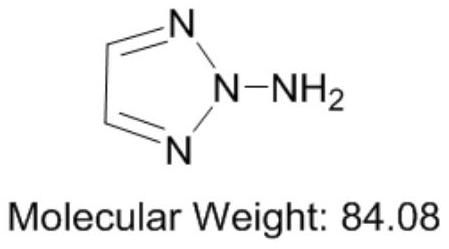
Synthesis method of 1-amino-1, 2, 3-triazole
A synthesis method and amino technology, applied in organic chemistry, electrolytic components, electrolytic organic production, etc., can solve problems such as low product yield, difficult separation of mixtures, active chemical properties, etc., to reduce pollutant emissions and reduce environmental pollution , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
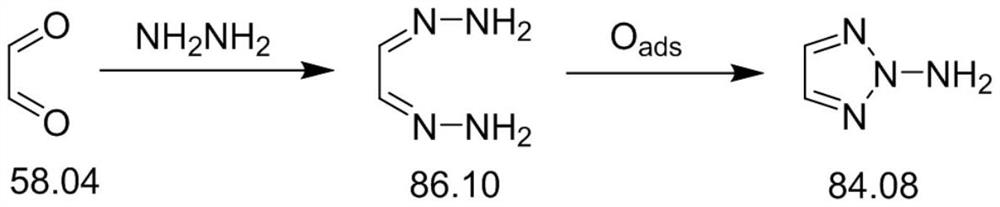
[0040] Preparation of Glycol:
[0041] Take 60 ml of methanol and 187.5 g (3.0 mol) of 80% hydrazine hydrate aqueous solution into a 500 ml three-necked flask, and control the temperature in an ice-water bath at 0-5°C. 145g (1.0mol) 40% glyoxal aqueous solution was slowly added dropwise within 20~30min, and the temperature was controlled at 0~10°C. A white precipitate was formed during the dropwise addition, and the reaction mixture was continuously stirred for 3h. Heating at 75°C, stirring until the precipitate just dissolves, concentrating under reduced pressure, standing at low temperature overnight and then cold filtration. Washed with isopropanol and dried in vacuo to obtain 78.45 g of white needle crystals. Recrystallization from acetonitrile gave 65.56 g (97%) of glyoxal bishydrazone with a total yield of 80.5%.
Embodiment 2
[0043] Preparation of 1-amino-1,2,3-triazole:
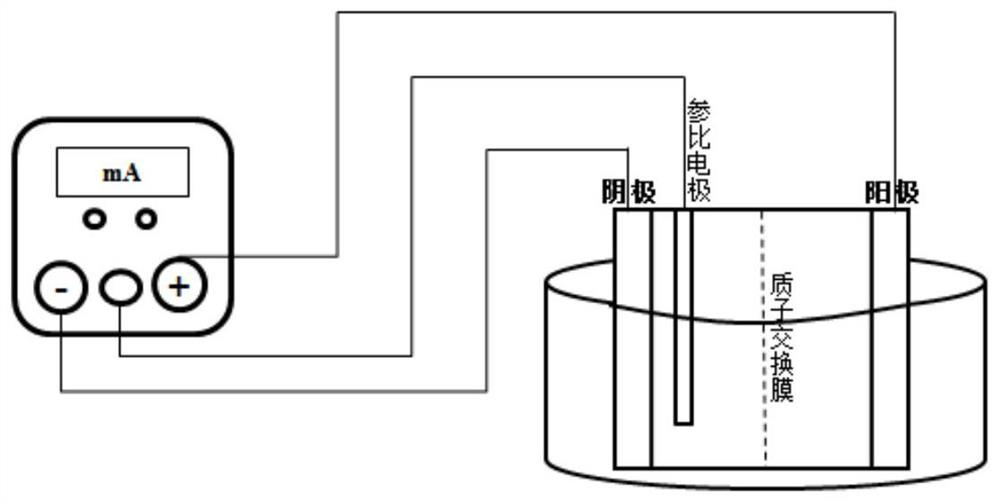
[0044] Reactor such as figure 1 As shown, the electrolytic cell is separated into an anode electrolytic cell and a cathode electrolytic cell by a proton exchange membrane, and the anode electrolytic cell and the cathode electrolytic cell are respectively connected with the positive and negative electrodes of the power supply through wires, and a reference electrode is also provided in the cathode electrolytic cell. The electrode stabs the lead wire to the power supply.
[0045] Get the glyoxal dihydrazone 25.0g and 500ml mass concentration that embodiment 1 prepares and be that the sodium carbonate of 2% joins in the electrolysis anode cell, as the reaction raw material and the electrolyte of anode electrolysis, take by weighing 0.1g MnO again 2 Added to the anode cell as a catalyst. The mass concentration of 1% NaOH solution was used as the catholyte, energized, and the current density was adjusted to 100mA / dm 2 , normal pres...
Embodiment 3
[0047] Preparation of 1-amino-1,2,3-triazole:
[0048] The reaction device adopted is the same as in Example 2;
[0049] Get the glyoxal dihydrazone 30.0g and 500ml mass concentration of the preparation of embodiment 1 and be 2% sodium chloride solution and join in electrolysis anode cell, as the reaction raw material and electrolyte of anode electrolysis, take by weighing 0.15gCuO again and add into anode cell as a catalyst. The mass concentration of 1% NaOH solution was used as the catholyte, energized, and the current density was adjusted to 100mA / dm 2, normal pressure, temperature of 30 ℃, electrolysis reaction for 8.0h, the reaction is over, activated carbon decolorization and filtration, concentrated under reduced pressure and then placed at low temperature to precipitate crystals, cold filtration and washing with chloroform to obtain white crystals, the mother liquor is concentrated again, left standing at low temperature and cold filtration and After washing, a small...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com