Red light emitting material based on aza-beta diketone boron difluoride central core, preparation method and application thereof, and organic electroluminescent device
A boron difluoride and diketone technology, which is applied in the field of organic electroluminescence devices, can solve the problems of low fluorescence quantum yield of red light materials, difficulty in realizing deep red light emission, restricting the application of red light materials, etc., so as to improve the luminescence Efficiency, realizing commercial application, and the effect of simple operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The present invention provides the preparation method of the red light material described in the above scheme, comprising the following steps:
[0044] (1) Put R 1 H, amide solvent, 4-fluorobenzamide and potassium tert-butoxide are mixed, carry out the first nucleophilic substitution reaction, obtain the compound of the structure shown in formula C;
[0045] (2) mixing the compound of the structure shown in the formula C, methyl p-halogenated benzoate, a polar solvent and sodium cyanide, and carrying out the second nucleophilic substitution reaction to obtain the compound of the structure shown in the formula D;
[0046] (3) the compound of the structure shown in the formula D, boron trifluoride ether, piperidine and the chlorinated alkane solvent are mixed, carry out Knoevenagel condensation reaction, obtain the compound of the structure shown in the formula E;
[0047] (4) The compound of the structure represented by the formula E, R 2 H, tris(dibenzylideneacetone)d...
Embodiment 1
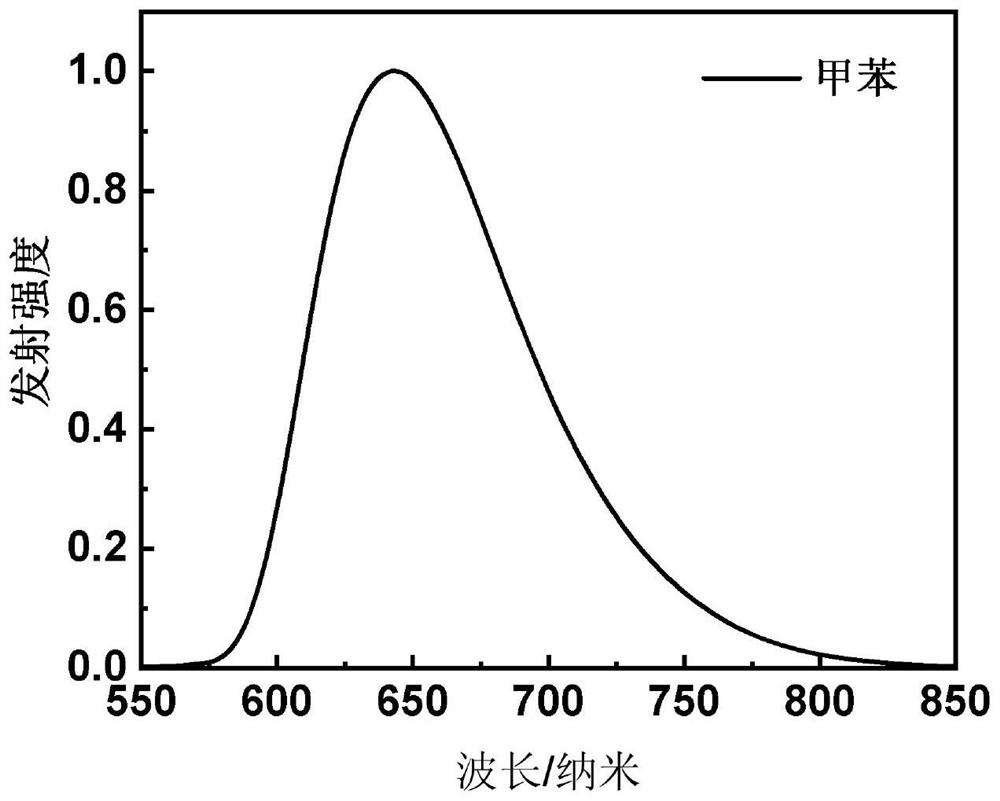
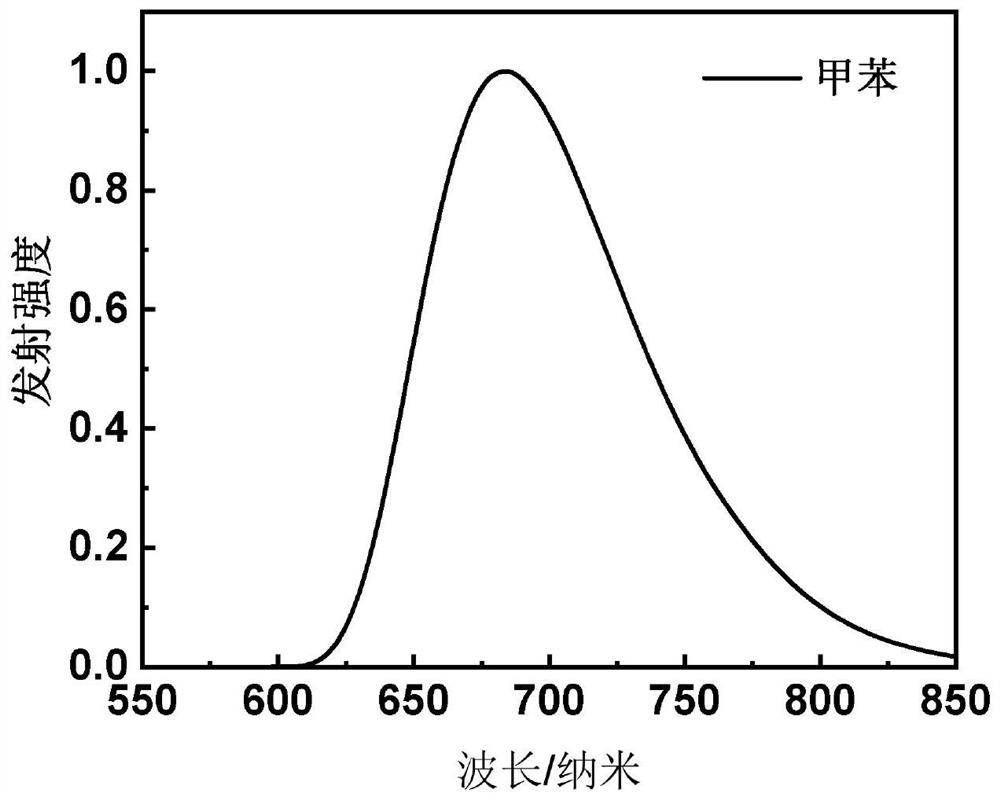
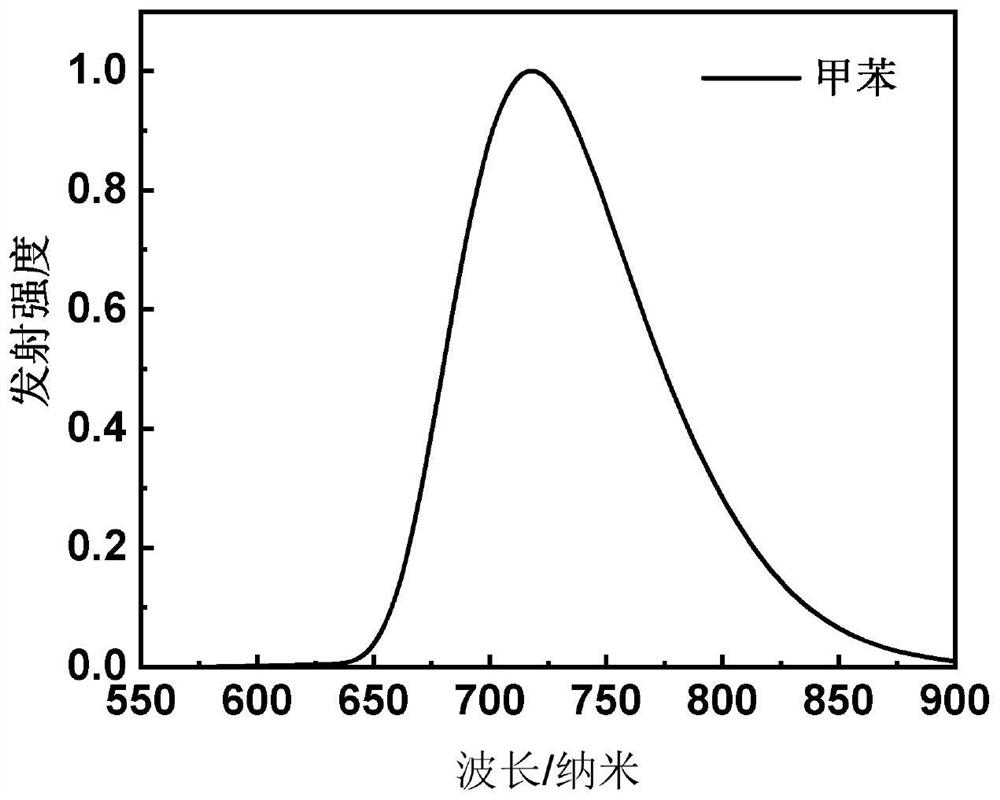
[0089] This embodiment provides a red light material based on the central core of aza-β-diketone boron difluoride, which has the structure shown in the following formula I-1:
[0090]
[0091] (1). The synthetic method of compound triphenylamine benzamide (C):
[0092]
[0093] Diphenylamine (16.7 g, 100 mmol) was dissolved in N,N-dimethylformamide (100 mL), to which were added 4-fluorobenzamide (13.9 g, 100 mmol) and potassium tert-butoxide (5.6 g, 50 mmol) ), vacuumed and passed nitrogen for three times, heated to 110°C, and stirred for 24 hours. Cool to room temperature, add the reactant to 400 mL of water, a white solid will be precipitated, use CH 2 Cl 2 Extraction was followed by washing with saturated NaCl solution, drying over anhydrous magnesium sulfate, and filtration to obtain the organic phase. The organic solvent was evaporated under reduced pressure, and the obtained crude product was purified by column chromatography (petroleum ether:ethyl acetate=12:1 ...
Embodiment 2
[0104] This embodiment provides a red light material based on the central core of aza-β-diketone boron difluoride, which has the structure shown in the following formula I-2:
[0105]
[0106] The only difference from Example 1 is that diphenylamine in step (1) and step (4) is replaced with carbazole to obtain azafluoroboron dipyrrole red light compound I-2 (18.75g, 31.08mmol, 60% yield). Structural Characterization Data: 1 HNMR (600MHz, CDCl 3 )δ8.43-8.40(m, 4H), 8.20(dd, J=8.4, 1.2Hz, 2H), 8.16-8.14(m, 2H), 7.86-7.82(m, 4H), 7.73(t, J= 7.4Hz, 2H), 7.61-7.58(m, 2H), 7.54(d, J=8.2Hz, 2H), 7.47-7.44(m, 2H), 7.37-7.33(m, 2H), 7.27(s, 2H) ); the molecular ion mass determined by mass spectrometry was: 603.20 (calculated value: 603.19).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com