Catalase mutant and application thereof
A catalase and mutant technology, applied in the field of catalase mutants and their preparation, can solve problems such as insufficient research on enzymatic characteristics and catalytic activity, and achieve improved molar conversion rate of products and high industrialization and application prospects, the effect of improving enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Construction of initial catalase gene recombinant Escherichia coli
[0041] 1. For the catalase gene derived from Bacillus mojavensis LDFZ001, our laboratory has constructed a recombinant vector pET28a-BmCAT derived from the catalase gene of Bacillus mojavensis LDFZ001 in the early stage, and its amino acid sequence is as shown in SEQ ID NO.1 shown.
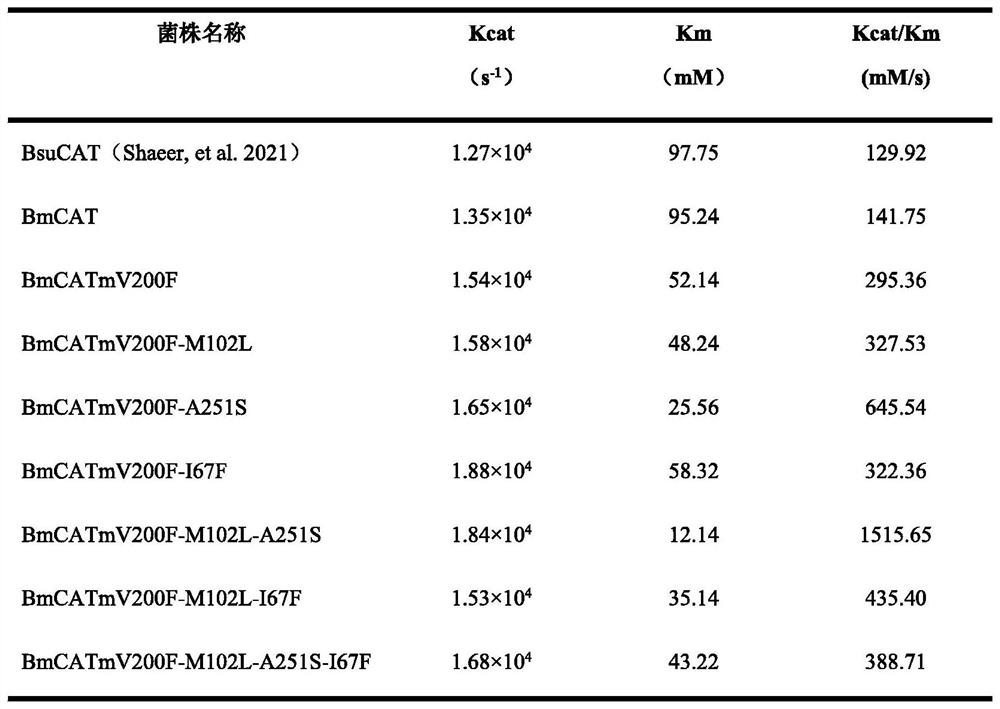
[0042] 2. For catalase Cat derived from Bacillus subtilis R5 Bsu , according to the amino acid sequence published in the literature (AbeeraShaeer, et al., Structural and functional analyses of a novel manganese-catalase from Bacillus subtilis R5.Int J Biol Macromol, 180(2021): 222-233), namely SEQ ID NO.3, Based on this, the whole gene synthesis was carried out by Huada Company, the synthetic gene sequence was SEQ ID NO.4, and restriction endonuclease sites BamH I and Xho I were introduced at both ends of the gene, catalase Cat Bsu The digested fragment was ligated with the digested fragment of the vector pET28...
Embodiment 2
[0044] Example 2 Construction of random mutant library of initial catalase BmCAT by error-prone PCR
[0045] 1. Using the original catalase recombinant plasmid pET28a-BmCAT as a template, a random mutant library was constructed by error-prone PCR technology. The error-prone PCR kit was purchased from Clontech (Clontech, PT3393-1); the single-point mutation kit was purchased from Nanjing Novizan Biotechnology Co., Ltd. (Novizan, C214).
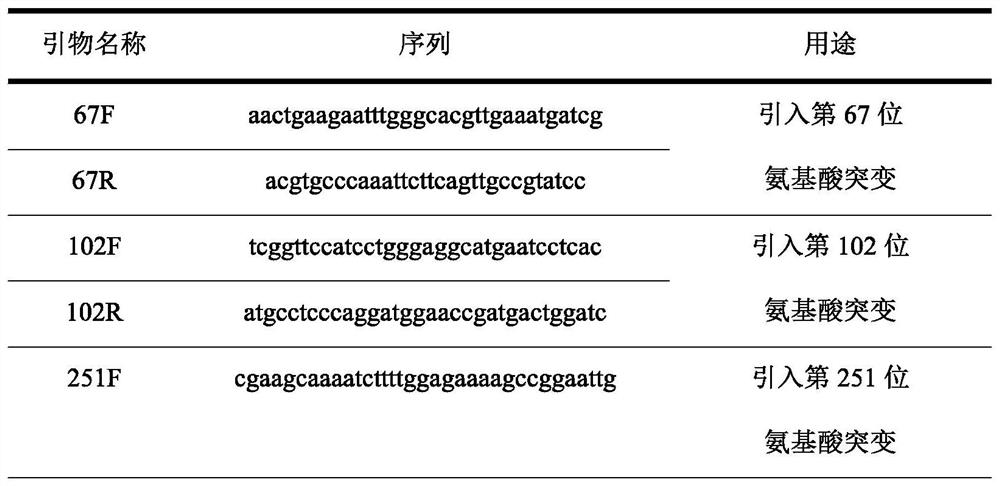
[0046] 2. The error-prone PCR kit (Clontech, PT3393-1) was used to carry out error-prone PCR amplification with plasmid pET28a-BmMnCAT as template and BmCAT-F and BmCAT-R as primers. The primer sequences are as follows:
[0047] BmCAT-F: acagcaaatgggtcgcggatccATGTTTAAACATACGAAAATGC
[0048] BmCAT-R: gtggtggtggtggtggtgctcgagTTACTCACGCCCAGGAAGCG
[0049] where lowercase letters are homology arm sequences.
[0050] The PCR reaction system is 50 μL, template DNA (final concentration is about 1 ng / μL) 1 μL, forward primer (10 nM) and reverse pri...
Embodiment 3
[0054] Example 3 High Throughput Screening of Mutant Libraries
[0055] 1. Preparation of Mutant Crude Enzyme Liquid Wet Bacteria
[0056] The mutant recombinant Escherichia coli monoclone obtained in Example 2 was picked into a sterile 96 deep-well plate, and each well contained 1 mL of LB liquid medium containing 50 μg / mL kanamycin, and cultured overnight at 37°C and 180 rpm. 8h, then transfer 500 μL of bacterial liquid at a ratio of 1:1 to another LB liquid medium containing 500 μL of kanamycin and a final concentration of 0.2 mM IPTG in each well, and continue to culture at 20 °C and 180 rpm. After 16 hours, the cells were collected by centrifugation at 8000 rpm at room temperature for 5 min to obtain 543 recombinant Escherichia coli wet cells containing mutant genes, namely mutant wet bacteria.
[0057] 2. Primary screening
[0058] The catalase activity detection was carried out using a catalase test kit (Sangon Biotechnology, D7995598-0100).
[0059] Standard enzyme ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com