Polymer nano-micelle entrapped with two drugs and preparation method and application of polymer nano-micelle
A nano-micelle and polymer technology, applied in the field of biomedicine, can solve the problems of cumbersome design and preparation of micelles, difficult realization of chemotherapy drugs, and increased difficulty of research, etc., to achieve excellent clinical application prospects, controllable drug release behavior, Inhibition of tumor growth and metastasis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1. Preparation of polymer nanomicelles that simultaneously encapsulate two drugs according to the present invention
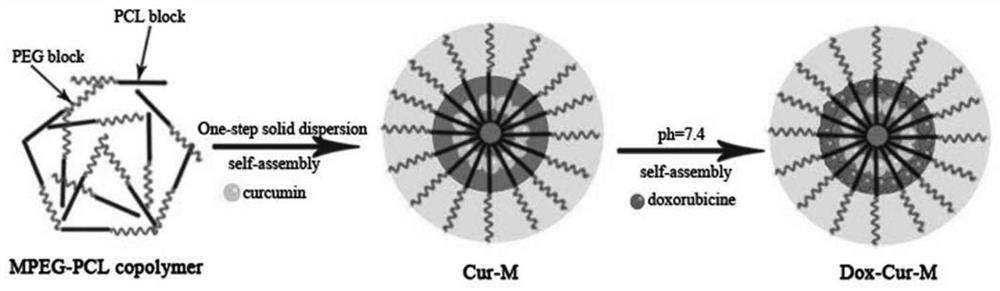
[0071] The mPEG-PCL polymer nanomicelles (Dox-Cur-M) loaded with doxorubicin and curcumin simultaneously were obtained in two steps.
[0072] Step 1: Preparation of curcumin-encapsulated mPEG-PCL micelles (Cur-M) by solid dispersion method: Curcumin and mPEG-PCL polymer were weighed and added to 5 mL of ethanol to dissolve completely. Then use a rotary evaporator to remove ethanol by rotary evaporation for 10 minutes at 60°C, rotating speed of 100rpm / min and negative pressure. After forming a transparent film, add distilled water and place it in a water bath for hydration at 60°C to obtain Cur-M micelle solution. , the curcumin concentration in the solution is 1.5mg / mL, and the mPEG-PCL polymer concentration is 60mg / mL
[0073] Step 2: Encapsulation of doxorubicin into Cur-M micelles by pH-induced self-assembly. First, 0.1 mL of PBS (10×, pH 7....
experiment example 1
[0079] Experimental Example 1. Characterization of physicochemical properties of Dox-Cur-M micelles of the present invention
[0080] 1. Experimental method
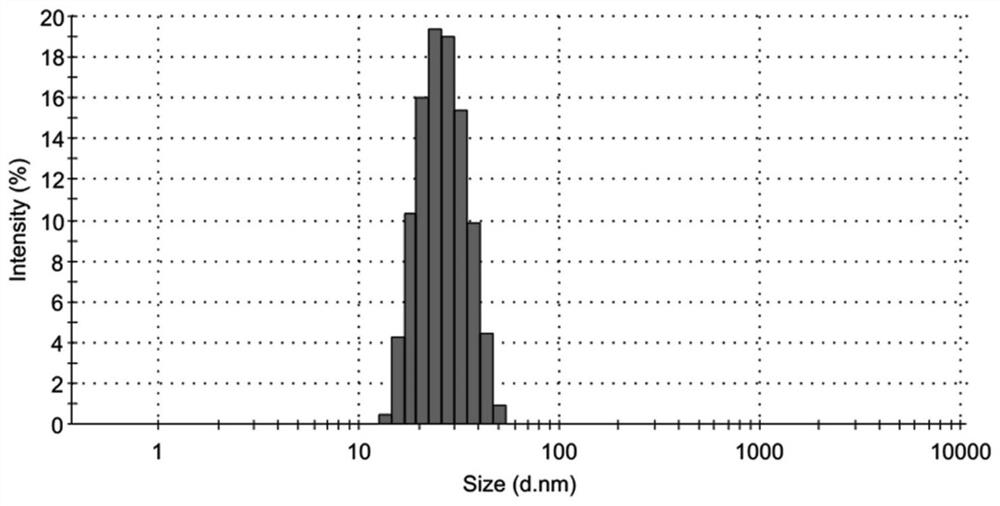
[0081] 1.1 Determination of particle size of Dox-Cur-M micelles
[0082] All Dox-Cur-M micelles tested below are Dox-Cur-M micelles prepared in Example 1.
[0083] The hydrated particle size and distribution of Dox-Cur-M micelles were measured by dynamic light scattering (DLS) using a laser scattering particle sizer (Nano-ZS, Malvern Instrument, UK); the samples were tested at 25°C and the test was repeated 3 times, take the average value.
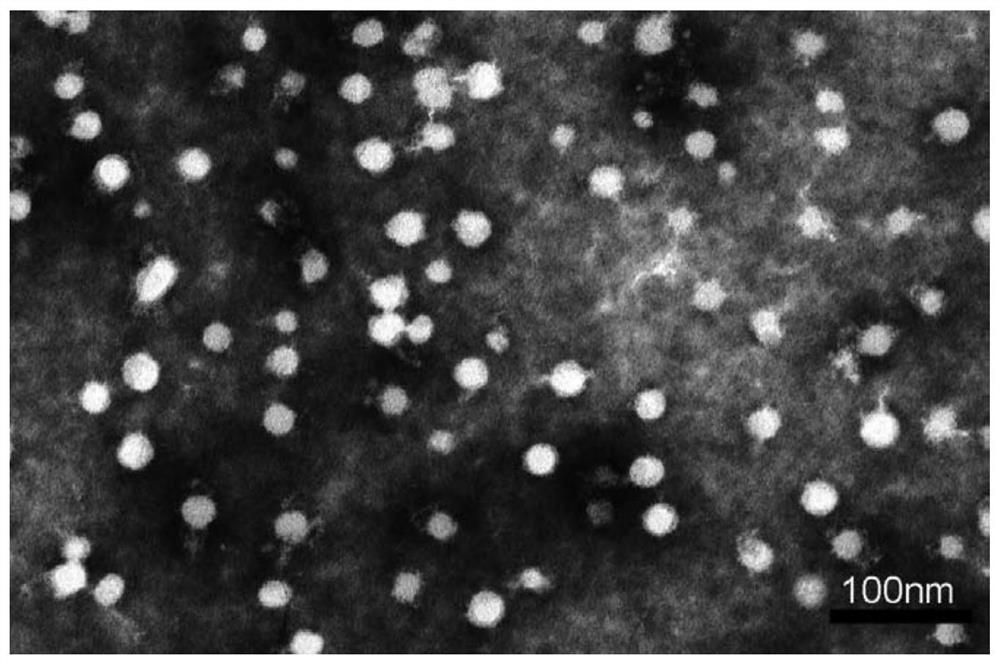
[0084] 1.2 Morphology of real Dox-Cur-M micelles observed by transmission electron microscopy
[0085] The morphology of Dox-Cur-M micelles was observed using transmission electron microscopy (TEM). Before observation, a drop of diluted Dox-Cur-M micelles of Example 1 was dropped onto a copper mesh, then negatively stained with phosphotungstic acid solution, dried and placed unde...
experiment example 2
[0093] Experimental example 2. Establishment of HPLC analysis method and determination of encapsulation efficiency and drug loading
[0094] 1. Experimental method
[0095] 1.1 Chromatographic conditions
[0096] Waters Alliance 2695 high performance liquid chromatograph, chromatographic column Inertsil / WondaSil C18 (4.6x250mm, 5um), column temperature 30°C, injection volume 20μL, flow rate: 1.0mL / min, the mobile phase for curcumin detection is acetonitrile: 1% acetic acid solution (60 / 40, V / V), detection wavelength 420nm; mobile phase for doxorubicin detection is methanol: acetonitrile: 1% acetic acid solution (40 / 10 / 50, V / V), detection wavelength 252nm.
[0097] 1.2 Standard curves of curcumin and doxorubicin
[0098] Precisely weigh 10 mg of curcumin using an electronic scale, place it in a 100 mL volumetric flask, and add 100 mL of methanol to dissolve it to obtain a stock solution. The methanol standard solutions of curcumin with different concentrations of 0.1, 0.4, 0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| cumulative release rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com